Researchers Develop Simplified Mass Spectrometry Approach for Neutrophil Study
Neutrophil granulocytes, a crucial component of the immune system, play a vital role in defending against pathogens. However, their infiltration into injured tissue—such as the brain following a stroke—can exacerbate chronic inflammation and contribute to long-term damage. To better understand their behavior, researchers at ISAS, the University Hospital Essen, and the University of Münster have developed a novel method to analyze neutrophil proteins in inflamed tissues using mass spectrometry, requiring just 1,000 cells.
A Breakthrough in Immune Cell Analysis
Traditional proteomic studies necessitate millions of neutrophils, posing a challenge as inflammatory sites often contain only a limited number of these cells. The newly developed method allows for the examination of individual inflammatory foci, enhancing the precision of immune response analysis. The findings, published in Molecular & Cellular Proteomics, include a freely accessible database and a neutrophil proteome web service.
By refining protein digestion techniques and optimizing liquid chromatography-mass spectrometry (LC-MS) acquisition, the researchers built extensive spectral libraries using neutrophils from five healthy humans and five healthy mice. These datasets, encompassing approximately 5,300 human and 6,200 mouse proteins, provide a valuable reference for future studies.
Tabasco Sauce Validates the Method
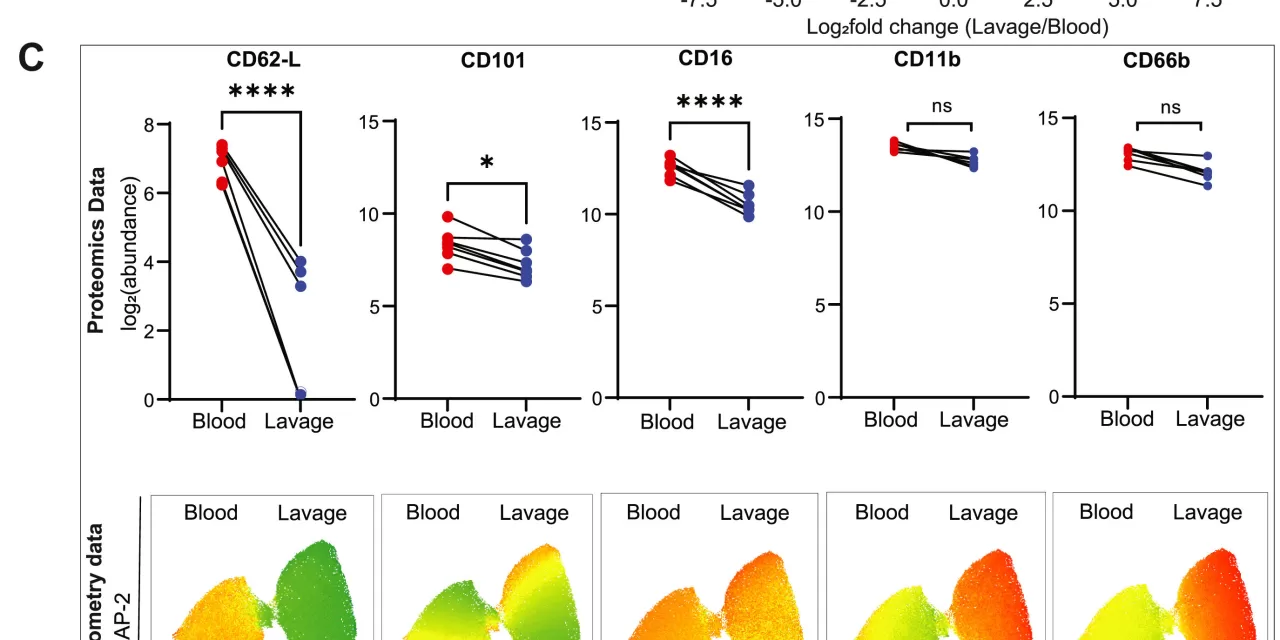
To test the reliability of their approach, the scientists examined neutrophils under two distinct conditions. First, they studied neutrophils in mouse brains following a stroke, where the cells displayed increased mitochondrial activity in response to a low-glucose environment. Next, they investigated human neutrophils triggered by a unique method—mouth rinsing with a Tabasco-infused solution.
At the University of Münster, seven participants swished a solution containing Tabasco sauce, inducing transient oral inflammation. This prompted neutrophils to migrate from the bloodstream to inflamed tissue, mirroring natural immune responses. The researchers confirmed that these transmigrated cells underwent functional changes, verified through flow cytometry.
Future Implications and Advancements
The study demonstrates that the new method, coupled with spectral library data, is applicable across various inflammation models. Lead author Susmita Ghosh, a Ph.D. student at ISAS, highlighted the potential of further refining the technique. “In the long-term, we aim to enable proteomic analyses for a handful of neutrophil numbers, even down to individual cells,” she stated, citing initial promising results using as few as five cells.
This innovative approach not only enhances the specificity of neutrophil analysis but also conserves biological sample resources, particularly beneficial in reducing the use of laboratory animals. The publicly accessible spectral libraries will serve as a vital resource for future immunological research.
For more details, refer to the original study: Susmita Ghosh et al., Molecular & Cellular Proteomics (2024). DOI: 10.1016/j.mcpro.2024.100858
[Disclaimer: This article is for informational purposes only and does not constitute medical advice.]