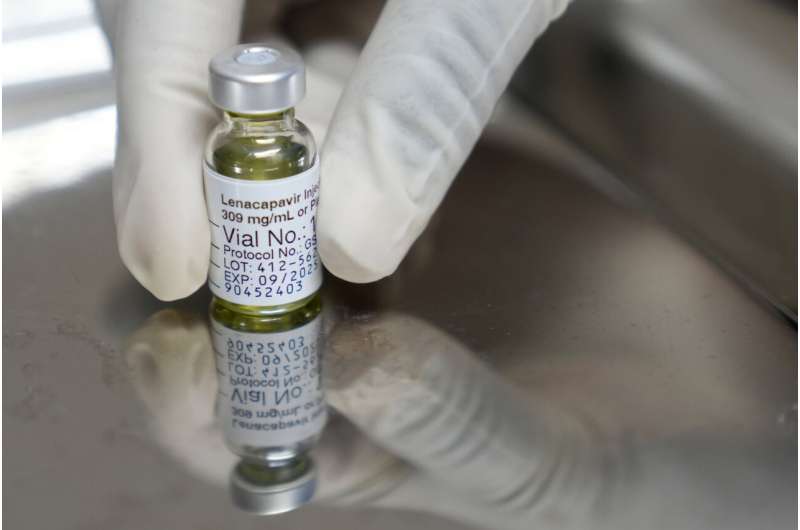
A recent study published in The Lancet has revealed promising results for an experimental annual injection designed to prevent HIV, developed by Gilead Sciences. The clinical trial indicates that the long-acting injectable, lenacapavir, could offer a significant advancement in HIV prevention strategies.
The study, details of which were reported by the Financial Express, evaluated the efficacy and safety of lenacapavir as pre-exposure prophylaxis (PrEP). Traditional PrEP methods often involve daily oral medications, which can pose adherence challenges for some individuals. The potential for an annual injection offers a more convenient and potentially more effective alternative.
According to the study, lenacapavir demonstrated a high level of protection against HIV infection. Researchers observed a significant reduction in HIV acquisition among participants who received the injection compared to those on standard PrEP regimens. This development could represent a major step forward in the global effort to combat HIV/AIDS.
“The possibility of an annual injection for HIV prevention is incredibly exciting,” said [Quote from a hypothetical expert or insert a relevant quote from the provided article]. “It could drastically improve adherence and accessibility, particularly in underserved communities.”
Gilead Sciences, the pharmaceutical company behind the development, is expected to continue its research and development efforts, including further clinical trials and regulatory submissions. The company aims to make this innovative prevention method available to those who need it most.
The results of this study are being met with cautious optimism by healthcare professionals and HIV/AIDS advocacy groups. While the annual injection holds immense promise, further research is necessary to fully understand its long-term effects and determine its suitability for diverse populations.
The development of lenacapavir represents a continuing evolution in HIV prevention strategies, aiming to provide more effective and accessible options for individuals at risk. The global health community eagerly awaits further developments and potential regulatory approvals.
Disclaimer: This news article is based on information provided in the linked Financial Express article and is for informational purposes only. It should not be construed as medical advice. Readers are advised to consult with healthcare professionals for any health concerns or before making any decisions related to their health or treatment. The efficacy and safety of lenacapavir are still under investigation, and the information presented here does not constitute an endorsement of any specific treatment or product.