Five years after the COVID-19 outbreak, scientists continue to investigate its long-term effects and explore new strategies for combating viral diseases. A groundbreaking study, involving an international team of researchers and a laboratory at Texas Tech University, may have uncovered a crucial genetic clue in this fight.
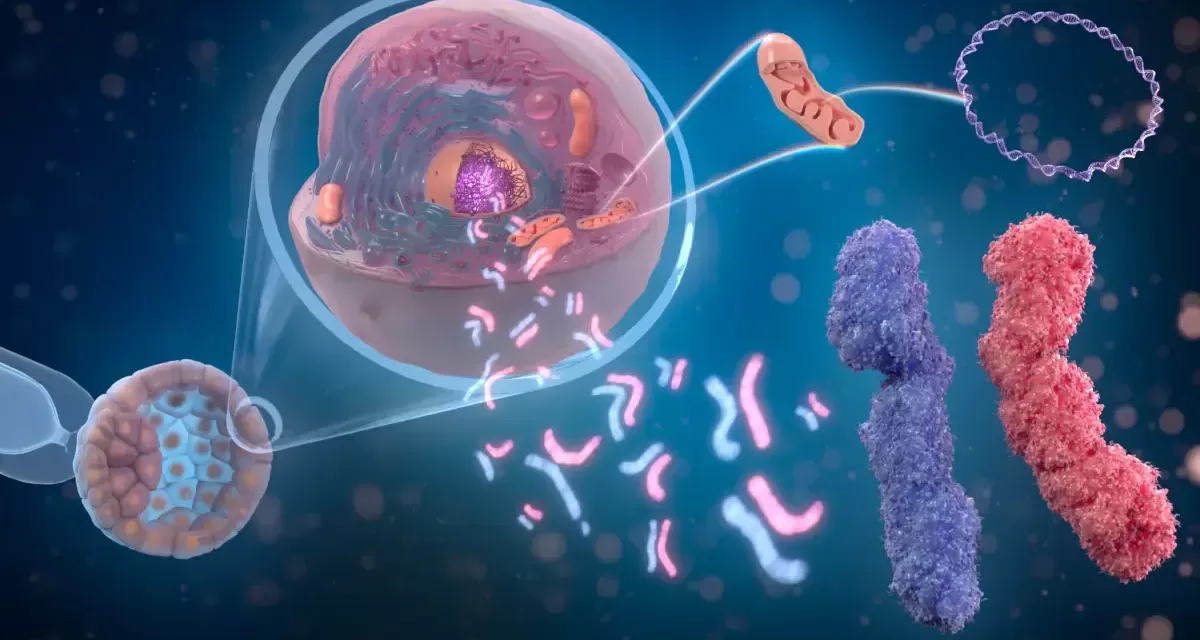
The Ray Laboratory, led by Professor and Associate Chair David Ray of Texas Tech’s Department of Biological Sciences, played a pivotal role in a recent study published in Nature on bat genomes. The research identified a genetic component in certain bat species that could significantly enhance immune responses against viruses, including SARS-CoV-2, the virus responsible for COVID-19.
According to the study, a specific gene found in bats, known as ISG15, can reduce SARS-CoV-2 production by up to 90%. This discovery could pave the way for new medical approaches to combating viral infections in humans.
“Bats have an amazing ability to resist some of the worst effects of viral infections that make humans so vulnerable,” said Ray. “While we get very sick, bats barely blink an eye when exposed to the same pathogens.”
Unlocking Genetic Defenses
Ray’s laboratory contributed to genome annotation, a process that identifies and characterizes genetic sequences, including coding and non-coding regions. The Texas Tech team focused on transposable elements (TEs)—sequences of DNA that can move within the genome and contribute to genetic diversity.
“Bats have a unique TE repertoire among mammals,” Ray explained. “These elements may provide a powerful way to generate new genetic pathways for dealing with pathogens like the coronavirus.”
Ray emphasized the importance of genetic diversity in combating infectious diseases: “If every individual of a species were genetically identical, they would all face the same risk of infection. TEs help create genetic variability, ensuring that some individuals are more resistant to diseases.”
A Global Scientific Collaboration
The study is part of the Bat1K project, an international initiative aiming to sequence and assemble the genomes of all 1,500 bat species. Led by the Senckenberg Research Institute and Natural History Museum in Frankfurt, Germany, the project involves leading scientists from around the world, including Michael Hiller, a professor of comparative genomics at Goethe University.
Hiller, a member of the Bat1K executive board alongside Ray, noted that the study’s findings could have profound implications for future antiviral research. “The ISG15 gene is likely one of several factors that contribute to bats’ resistance to viral diseases,” he said. “These promising results provide a foundation for further experimental studies to understand the unique adaptations of the bats’ immune system.”
Implications for Human Health
Unlike humans, bats can carry numerous viruses, including those transmissible to people, without exhibiting symptoms. This resistance suggests that bat immune systems have evolved unique mechanisms for viral defense, some of which may hold the key to developing new antiviral treatments.
The study demonstrated that the ISG15 gene from bats can significantly suppress SARS-CoV-2 replication, while the human version of the gene did not exhibit the same antiviral effect. Scientists believe that further research into bat immune adaptations could lead to novel therapeutic strategies to enhance human resistance to viral infections.
Ray’s laboratory has previously collaborated with organizations such as the National Science Foundation, the U.S. Department of Agriculture, and the Texas Department of Wildlife and Fisheries. Their research on bat genomes aligns with a broader effort to understand how genetic factors influence disease resistance across species.
Future Research and Potential Applications
The findings open the door for future studies to explore how bat genetics can be harnessed for medical advancements. Scientists are particularly interested in how transposable elements and immune-related genes can be leveraged to improve human defenses against viruses.
“Our work highlights the importance of genetic research in understanding disease resistance,” Ray said. “By studying how bats have evolved to combat infections, we may uncover new ways to protect human health.”
Disclaimer
This article presents findings from a scientific study and should not be interpreted as medical advice. Further research and clinical trials are necessary before any potential treatments based on this study can be developed for human use.