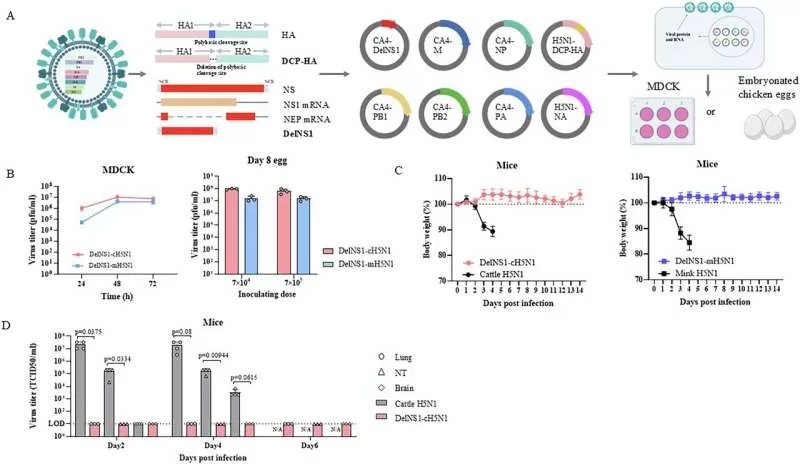
Hong Kong – In a significant stride towards pandemic preparedness, researchers at the University of Hong Kong (HKU) and the InnoHK Center for Virology, Vaccinology and Therapeutics (CVVT) have developed a novel nasal spray vaccine targeting the H5N1 avian influenza virus. This breakthrough, detailed in a study published in Nature Communications, leverages an influenza virus vector-based platform previously successful in creating a nasal COVID-19 vaccine.
The development comes at a critical time, as the world grapples with the lingering effects of the COVID-19 pandemic and the looming threat of new viral outbreaks. The World Health Organization (WHO) and global health agencies are particularly concerned about the H5N1 avian influenza virus, which has recently shown signs of increased transmissibility among mammals, including cattle in the United States.
“The H5N1 virus, first detected in humans in Hong Kong in 1997, has evolved into numerous variants and demonstrated cross-species infection capabilities, making it a prime candidate for a future pandemic,” researchers emphasized. Recent outbreaks in U.S. dairy farms, coupled with the identification of mutations enhancing the virus’s affinity for human respiratory cells, have heightened concerns about a potential pandemic.
Lessons learned from the COVID-19 pandemic highlight the crucial role of rapidly deployable and effective vaccines. However, current intramuscular vaccines, while effective in preventing severe disease, often fail to curb viral transmission due to limited mucosal immunity. The new nasal spray vaccine aims to address this critical gap.
“Our nasal spray H5N1 vaccine, developed using the established influenza virus vector platform, induces robust mucosal immunity at the primary site of viral entry, offering comprehensive protection,” the researchers stated. Animal studies have shown the vaccine’s high safety profile and its ability to elicit neutralizing antibodies, T-cell responses, and crucially, strong mucosal immunity in the upper respiratory tract, all with a single dose.
This single-dose approach is particularly advantageous, potentially streamlining outbreak control by providing rapid protection and sustaining long-term immune memory. Clinical trials are anticipated to further validate the vaccine’s efficacy and safety in humans. The nasal delivery mechanism also promises to limit viral transmission early in an outbreak, a crucial factor in pandemic containment.
The development of this nasal spray vaccine is a significant step forward in preparing for potential H5N1 outbreaks and underscores the importance of innovative vaccine platforms in pandemic preparedness. The United States has already allocated $500 million for mRNA-based vaccine development against H5N1, highlighting the global urgency to address this emerging threat.
More information: Ying Liu et al, Intranasal influenza virus-vectored vaccine offers protection against clade 2.3.4.4b H5N1 infection in small animal models, Nature Communications (2025). DOI: 10.1038/s41467-025-58504-z
Journal information: Nature Communications
Disclaimer: This news article is based on the provided information and should not be considered medical advice. The efficacy and safety of the nasal spray H5N1 avian influenza vaccine in humans have yet to be fully established through clinical trials. Readers should consult with healthcare professionals for accurate and up-to-date information regarding influenza and vaccinations.