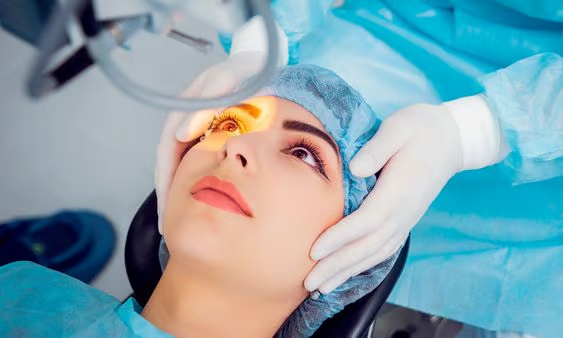
A groundbreaking stem-cell treatment has successfully restored vision in patients with severely impaired eyesight, marking a significant milestone in regenerative medicine. Four individuals with corneal damage, including two women and two men aged between 39 and 72, received stem-cell transplants that have improved their vision, with three patients experiencing long-lasting benefits. This pioneering treatment is the first of its kind to use reprogrammed stem cells to repair damaged corneas, the transparent outer layer of the eye.
The results, which were published today in The Lancet, have been hailed as an exciting development in the field of stem-cell research. The patients, who were enrolled in the study between June 2019 and November 2020, suffered from limbal stem-cell deficiency (LSCD)—a condition where the stem cells needed to maintain the cornea are depleted, leading to blindness. LSCD can result from eye trauma, autoimmune disorders, or genetic diseases, and currently available treatments have been limited and invasive.
The treatment was developed by Kohji Nishida, an ophthalmologist at Osaka University in Japan, and his research team, who used an alternative source of stem cells to create the corneal transplants. Instead of relying on traditional stem cells taken from a healthy donor’s eye, the researchers used induced pluripotent stem (iPS) cells. These cells were reprogrammed from blood cells into an embryonic-like state and then transformed into thin, transparent sheets of corneal epithelial cells, capable of rejuvenating damaged corneas.
The procedure involved scraping away the scar tissue from the cornea in one eye, then transplanting the epithelial sheets derived from the iPS cells onto the damaged eye, followed by a protective contact lens. The results were promising: after two years, all four recipients experienced improvements in their vision, with a reduction in the corneal damage caused by LSCD. The improvements persisted in three patients, although one experienced a slight reversal in their condition after one year.
“This is an exciting development,” said Kapil Bharti, a translational stem-cell researcher at the U.S. National Eye Institute. “The results show promise, but more patients need to be treated to fully understand the treatment’s potential and long-term effects.” In particular, researchers were relieved to see that the transplants did not result in tumors, a known risk of iPS cell treatments, and there was no sign of immune rejection, even in patients who did not take immunosuppressant drugs.
The vision improvements have sparked hope that iPS cell-based treatments could become a viable option for treating LSCD and other eye diseases. “These results suggest we are headed in the right direction,” said Bharti, noting that several other iPS-cell-based trials are underway globally. Nishida and his team plan to launch larger clinical trials in March 2025 to further assess the treatment’s safety and efficacy.
As the field of stem-cell therapy for eye diseases continues to evolve, experts are optimistic about the potential of these treatments to restore sight in individuals with otherwise irreversible vision loss. “This breakthrough could pave the way for future treatments that significantly improve the quality of life for patients with damaged corneas,” said Jeanne Loring, a stem-cell researcher at Scripps Research in California.
The results provide hope for patients with severe corneal damage and underscore the importance of continued research into regenerative therapies. The world is watching closely as these innovative treatments progress, and the future of stem-cell-based vision restoration looks brighter than ever.
Source: The Lancet 2024. doi: 10.1038/d41586-024-03656-z.