August 10, 2024 – Geneva, Switzerland: The World Health Organization (WHO) has issued an urgent invitation for manufacturers of mpox vaccines to submit an Expression of Interest (EOI) for Emergency Use Listing (EUL). This announcement follows the WHO Director-General’s declaration on August 7, 2024, triggering the EUL process in response to the growing concerns about the spread of mpox.
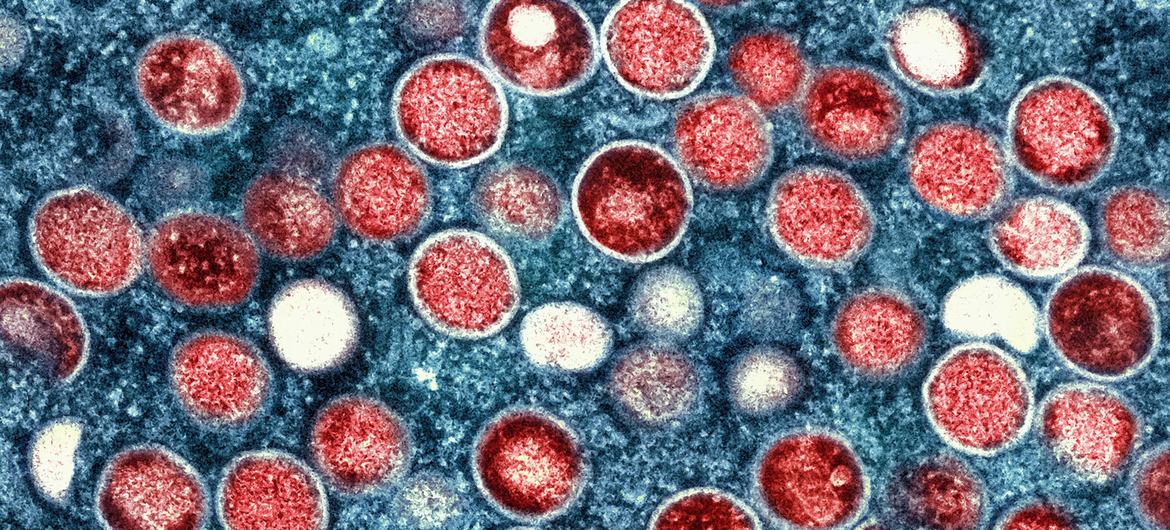
The current outbreak in the Democratic Republic of the Congo (DRC) has intensified, with the emergence of a new viral strain detected for the first time outside the DRC. This development underscores the critical need for swift action to control the disease, which poses a significant threat to global public health.
The EUL is a specialized emergency use authorization process designed to expedite the availability of unlicensed medical products, including vaccines, during public health emergencies. It is a time-limited recommendation that balances the risks and benefits to ensure that the vaccines are safe, effective, of assured quality, and suitable for the populations at risk.
By granting an EUL, WHO aims to accelerate vaccine access, particularly for lower-income countries that have not yet issued their own national regulatory approvals. The EUL also enables international partners, including Gavi, the Vaccine Alliance, and UNICEF, to procure and distribute vaccines more rapidly, ensuring that the most vulnerable populations are protected.
Mpox, caused by the monkeypox virus—a species of the Orthopoxvirus genus—remains a significant public health challenge. The virus can be transmitted to humans through direct contact with an infected person, contaminated materials, or infected animals.
Currently, two vaccines are recommended by the WHO Strategic Advisory Group of Experts on Immunization (SAGE) for use against mpox. These vaccines have played a crucial role in managing outbreaks, but the recent developments necessitate additional options and broader accessibility to curb the disease’s spread.
As WHO mobilizes resources and coordinates international efforts to combat this outbreak, the prompt submission of dossiers by vaccine manufacturers will be critical in the global response to mpox. The organization remains committed to ensuring that safe and effective vaccines are available to all who need them, particularly in regions most affected by the ongoing crisis.
This initiative reflects WHO’s ongoing commitment to addressing public health emergencies with urgency and precision, ensuring that no country is left behind in the fight against mpox.