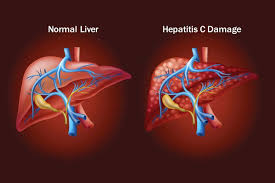
Many low- and middle-income countries have reduced suffering from hepatitis C, thanks to increased access to testing and treatment. Some achieved a 20-fold increase in the number of people treated with safe and effective direct-acting retroviral drugs between 2015 and 2018, according to the Global progress report on accelerating access to hepatitis C diagnostics and treatment, released by WHO today.
A drop in prices underpins this success
Low- and middle-income countries can now aim to achieve a price as low as US$ 60 per patient for a 12-week course of treatment with WHO-prequalified generic sofosbuvir and daclatasvir. Prices offered by suppliers of WHO-prequalified HCV rapid diagnostic tests ranged between US$ 1 and US$ 8 per test.
By 2018, more than 120 countries had adopted a national viral hepatitis strategy, up from 20 countries in 2012. This has accelerated since the adoption of WHO’s first Global Health Sector Strategy on Viral Hepatitis 2016–2021, with a number of countries making impressive progress to scale up government commitment, draw up national strategic plans, simplify guidelines, as well as, increasing availability of cheaper, quality-assured diagnostics and treatment options.
Despite the challenges of the ongoing COVID-19 pandemic, the progress achieved to date is impressive yet fragile, and access to hepatitis C testing and treatment has not reached sufficient levels of coverage to attain the global goal of eliminating viral hepatitis as a major public health threat by 2030. Globally, at the end of 2017, only 5 million – or 7% – of the 71 (62–79) million people chronically infected with HCV had cumulatively received treatment with direct-acting antivirals.
“As countries continue to tackle the disease burden and service disruptions caused by the COVID-19 pandemic, it is critical to ensure that the recent momentum and gains in the response to hepatitis C are not lost.” said Dr Minghui Ren, WHO Assistant Director-General Universal Health Coverage Communicable and Noncommunicable Diseases. “Global efforts to scale up access to high-impact interventions for hepatitis C must be sustained and accelerated in the coming decade, as part of broader efforts towards universal health coverage.”
“The sources of quality-assured generic direct-acting antivirals and diagnostics are steadily increasing and prices continue to fall” said Dr Mariangela Simao, Assistant Director-General Access to Medicines and Health Products at WHO. “Yet many countries are not accessing these low prices. Greater market transparency together with guidance and sharing experiences from around the world give practical examples for countries and communities to expand access and address persisting barriers”.
Despite recent price drops, availability and affordability of diagnostics remain a major barrier to treatment scale-up in low- and middle-income countries. It will be vital to expand access to simple, affordable and quality-assured hepatitis C in vitro diagnostics so that countries can screen large numbers of people, identify patients in need of treatment and provide appropriate care.
“Accelerating universal access to hepatitis diagnostic tests and multi-disease diagnostic analysers that can be used at or near the point of care is essential” said Dr Meg Doherty, Director of the Department of Global HIV, Hepatitis and STI Programmes at WHO. “We will continue working with governments, technical partners, civil society and world experts, to foster decentralization, task-sharing, simplifications, and integration of screening and diagnostic services with existing infrastructure in countries.”