Read Time:1 Minute, 15 Second
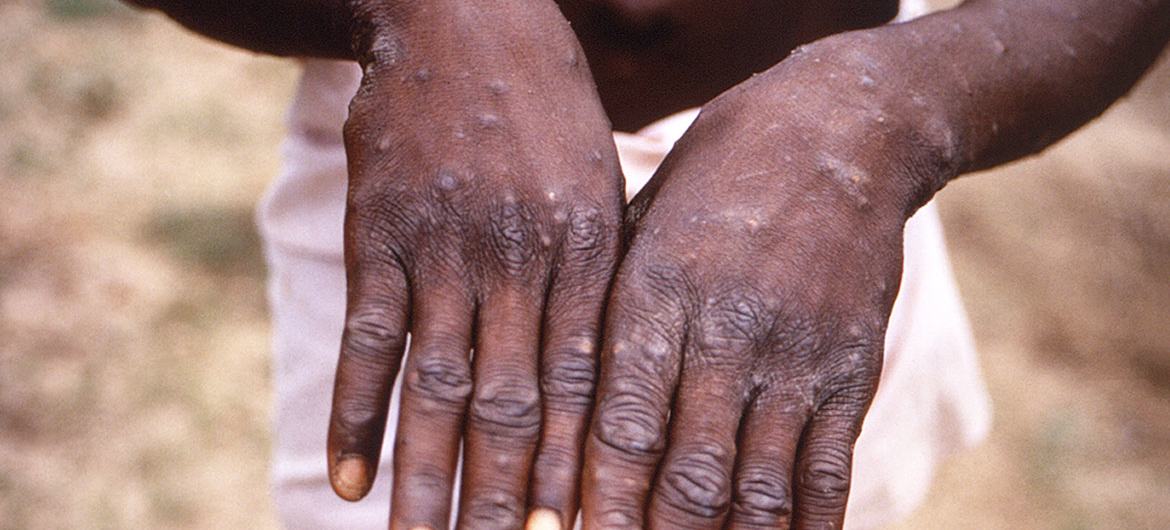
- On 14 June, WHO published a new vaccine and immunization guidance for monkeypox: https://www.who.int/publications/i/item/who-mpx-immunization-2022.1
- This interim guidance provides the first WHO recommendations on the use of (smallpox) vaccines for monkeypox.
- The goal of the global outbreak response for monkeypox is to control the outbreak and to effectively use public health measures to prevent the onward spread of the disease. Judicious use of vaccines can support this response.
- Based on currently assessed risks and benefits and regardless of vaccine supply, mass vaccination is not required nor recommended for monkeypox at this time.
- All decisions around immunization with smallpox or monkeypox vaccines should be by shared clinical decision-making, based on a joint assessment of risks and benefits, between a health care provider and prospective vaccinee, on a case-by-case basis.
- Control of monkeypox outbreaks primarily relies on public health measures including surveillance, contact tracing, isolation, and care of patients. While smallpox vaccines are expected to provide some protection against monkeypox, clinical data are limited.
- The supply of vaccines is limited and access strategies are under discussion.
- WHO is working closely with the Member States and partners to define what type of coordination mechanism could be put in place to ensure fair access to vaccines (and treatments)
- All efforts should be made to administer vaccines for monkeypox within a framework of collaborative research and randomized clinical trial (RCT) protocols with standardized data collection tools for clinical and outcome data.