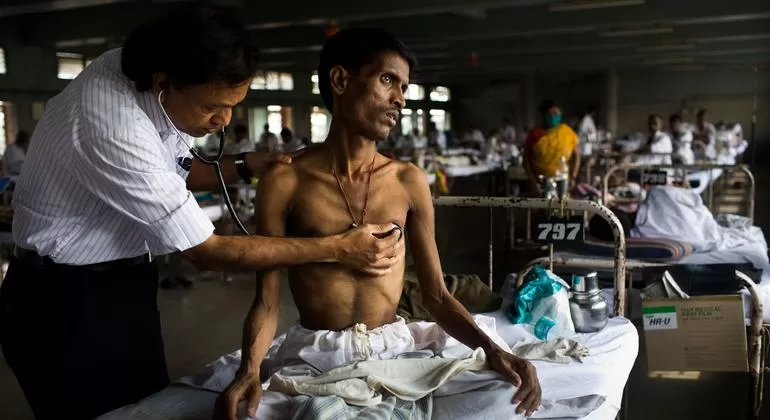
In a significant stride towards eliminating tuberculosis (TB), the Union Ministry of Health & Family Welfare has approved the introduction of a new, shorter, and highly efficacious treatment regimen for drug-resistant TB in India. The BPaLM regimen, consisting of four drugs—Bedaquiline, Pretomanid, Linezolid, and Moxifloxacin—has been shown to offer a quicker and more effective cure for patients suffering from Multi-Drug-Resistant Tuberculosis (MDR-TB).
This groundbreaking move aligns with India’s national goal of eliminating TB by 2025, five years ahead of the global target under the Sustainable Development Goals (SDGs). The announcement follows Prime Minister Narendra Modi’s vision to rid the country of TB and strengthen India’s public health landscape.
The BPaLM Regimen: A Game-Changer in TB Treatment
The newly introduced BPaLM regimen offers significant advantages over traditional MDR-TB treatments, which often span up to 20 months and are associated with severe side effects. In contrast, the BPaLM regimen promises a shorter, six-month course with a higher treatment success rate. This will greatly benefit India’s estimated 75,000 drug-resistant TB patients, providing a faster path to recovery and reducing healthcare costs.
Pretomanid, one of the key drugs in the regimen, has already been approved and licensed for use in India by the Central Drugs Standard Control Organization (CDSCO). The combination of Pretomanid with Bedaquiline, Linezolid, and Moxifloxacin has undergone rigorous validation by subject experts and health authorities to ensure its safety and cost-effectiveness.
Boosting India’s Progress in TB Elimination
The Union Government’s decision to introduce the BPaLM regimen is seen as a critical step in advancing India’s fight against TB. A country-wide roll-out plan is being developed by the Central TB Division in consultation with States and Union Territories. This plan will include intensive training for healthcare professionals to ensure the safe administration of the new treatment.
Health Minister Mansukh Mandaviya remarked that the shorter, more efficient regimen will drastically improve patient outcomes and contribute to achieving India’s TB elimination goal within the next year. “This approval represents not just a step forward in TB treatment but a leap in our commitment to end TB in India,” he said.
Background: India’s National TB Elimination Programme (NTEP)
India’s commitment to eliminating TB was first articulated at the Delhi End TB Summit in 2018. The National Tuberculosis Elimination Programme (NTEP), formerly known as the Revised National Tuberculosis Control Programme (RNTCP), has since been at the forefront of the country’s efforts to combat the disease. The NTEP covers over a billion people and is responsible for the Government of India’s five-year National Strategic Plans for TB elimination, in coordination with state and local governments.
The latest National Strategic Plan aims to detect all TB patients, particularly those in high-risk populations, and ensure that every diagnosed patient undergoes Universal Drug Susceptibility Testing (UDST) before beginning treatment.
In 2022, the Pradhan Mantri TB Mukt Bharat Abhiyaan was launched to further boost the fight against TB, with President Droupadi Murmu urging citizens to unite in their efforts. The Ni-kshay Mitra initiative, introduced under the Abhiyaan, aims to provide diagnostic, nutritional, and vocational support to TB patients, with a focus on community involvement and corporate social responsibility.
India’s Leading Role in Global TB Control
India houses the world’s largest TB laboratory network, with over 7,700 rapid molecular testing facilities and 87 culture and drug susceptibility testing laboratories. This extensive infrastructure will be pivotal in ensuring the timely detection of MDR-TB and the swift initiation of the new BPaLM regimen for those in need.
With the introduction of this innovative treatment, India is poised to make significant strides toward its ambitious goal of eradicating TB by 2025, demonstrating its leadership in global TB control efforts.