A world of microbes resides within the gut of every human being. This vast microbial community, known as the microbiome, comprises bacteria and viruses that actively contribute to both health and disease.
While researchers have extensively studied the bacterial communities in the gut—understanding their role in metabolizing food, shaping the immune system, and sometimes triggering disease—the influence of viruses remains less explored. A groundbreaking study published in Nature Microbiology has now shed new light on this aspect of the gut microbiome.
Phages and Their Role in Human Health
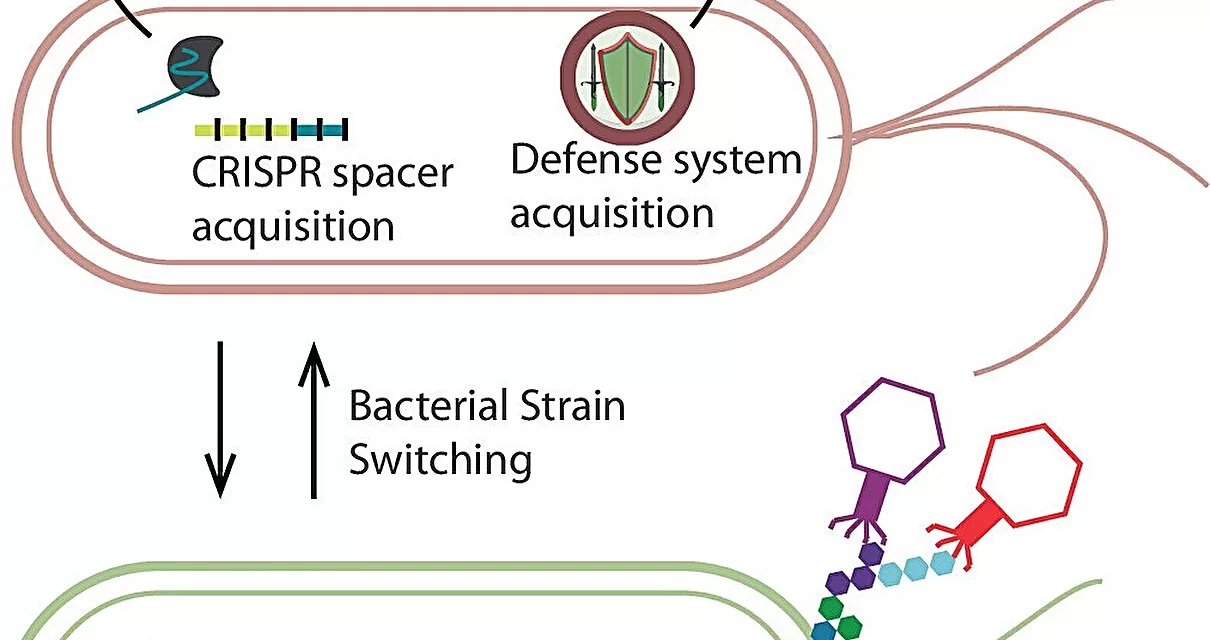
Scientists at Baylor College of Medicine have taken the lead in investigating whether certain viruses, called bacteriophages (or phages), which infect bacteria but not human cells, influence the development of type 1 diabetes in young children. Phages put pressure on the bacteria they infect, and some phage genomes encode virulence factors and toxins, suggesting they may play a role in human health.
“We think that phages can affect bacterial survival or behavior, and this, in turn, could influence human health,” said Dr. Michael J. Tisza, assistant professor of molecular virology and microbiology at Baylor and first author of the study.
The TEDDY Study and Microbiome Dynamics
The research team re-analyzed samples from The Environmental Determinants of Diabetes in the Young (TEDDY) study, which tracked children at risk of developing type 1 diabetes. While previous studies found an association between human viruses and diabetes but not gut bacteria, this new research aimed to specifically profile the interplay between phages and bacteria in the gut.
Analyzing over 12,000 stool samples from nearly 900 children during their first four years of life, the researchers discovered that bacterial communities shift in response to human development—and so do phages, but at a much faster rate. “This points to an ongoing arms race between bacteria and their phages, where bacteria evolve to escape predation, allowing new phages to emerge and infect them,” explained Tisza.
Implications for Future Research and Medicine
Although the team did not find major phage-related factors directly influencing type 1 diabetes risk, their findings provide a broader understanding of microbiome development. The study highlights how bacterial and viral interactions shape the gut environment and suggests that the immune system may experience more viral stimulation than previously thought.
“Understanding phage-bacteria interactions could lead to improved therapeutic approaches to disease,” said co-author Dr. Sara J. Javornik Cregeen.
The potential for microbiome manipulation as a medical intervention is growing. Dr. Joseph Petrosino, chair of molecular virology and microbiology at Baylor, emphasized that phage-based strategies could help manage diseases and combat antibiotic resistance, offering an alternative to traditional antibiotics.
As researchers continue exploring these relationships, they aim to understand how phages influence bacterial responses to external factors like antibiotics, diet changes, and new bacterial introductions. This study lays the foundation for future diagnostics and therapeutics targeting the microbiome.
Disclaimer:
This article is based on findings published in Nature Microbiology and aims to provide general information. It is not a substitute for professional medical advice, diagnosis, or treatment. Always seek guidance from a qualified healthcare provider regarding any medical condition.