Date: August 28, 2024
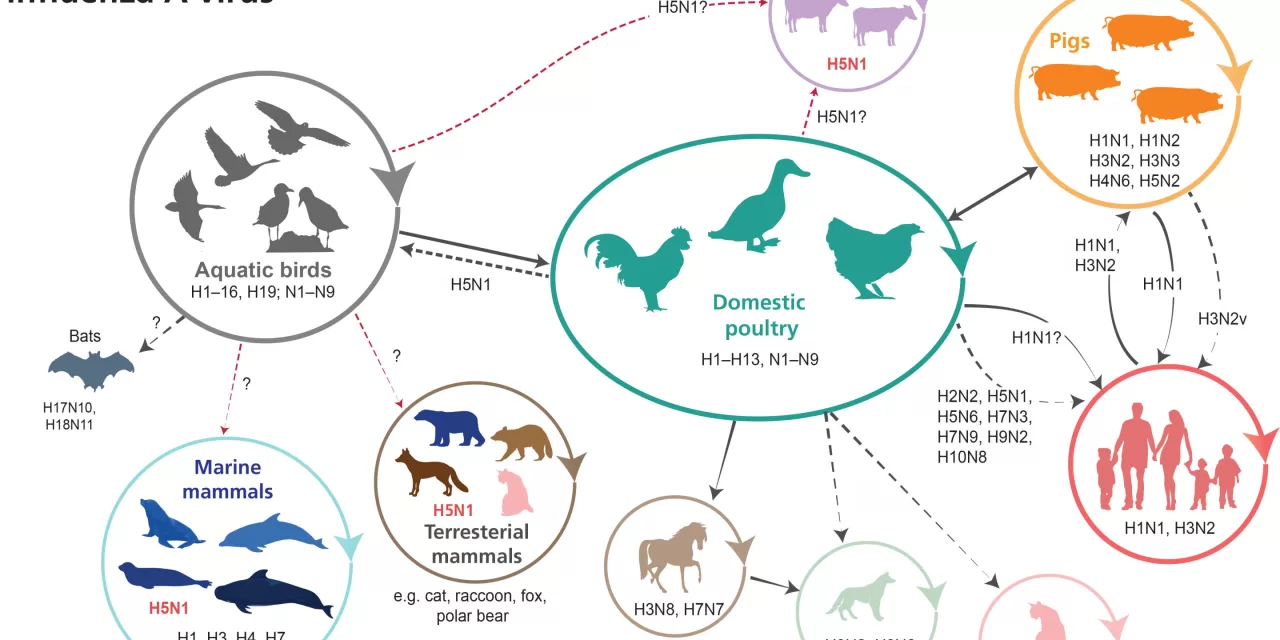
In the vast tapestry of life on Earth, viruses weave an intricate pattern that both fascinates and terrifies. Recently, this dance took a disconcerting turn as bird flu, or avian influenza, spread to an unexpected host: dairy cows.
The startling news emerged in March 2024, revealing that H5N1, a variant of bird flu typically confined to wild waterfowl, had infected dairy cows. “It was hard to believe,” remarked Yvonne Su, an evolutionary biologist with Duke-NUS’ Emerging Infectious Diseases Programme. Historically, cows were thought to be immune to avian influenza, making this outbreak particularly alarming.
Su’s initial concerns focused on whether the virus could spread directly between cows. By June, preliminary data suggested that the virus not only bound to the mammary glands in cows but also exhibited some degree of cow-to-cow transmission.
As the outbreak intensified, the scope of the epidemic became increasingly clear. By July, H5N1 had infected cows on 161 dairy farms across 13 US states, alongside poultry on 37 farms and nine humans. In Southeast Asia, a genetically distinct variant of H5N1 had resurfaced, causing outbreaks in poultry and infecting at least 13 humans in Cambodia, with additional cases reported in Vietnam and China.
“The recent surge in avian influenza outbreaks is deeply concerning,” said Kachen Wongsathapornchai, Regional Manager of the Food & Agriculture Organization’s Emergency Centre for Transboundary Animal Diseases. Wongsathapornchai emphasized the need for immediate, coordinated preventive measures to address the spread of novel A/H5N1 strains.
Influenza’s Evolutionary Dance
Influenza viruses, known for their high transmissibility, have coexisted with humans for centuries. Influenza A, the most pervasive type, infects a wide range of species—from birds to polar bears, and now, cows. Historically, all 14 influenza pandemics have stemmed from influenza A viruses.
H5N1, though not yet causing a pandemic-scale outbreak in humans like H1N1, has made a notable impact. The virus first shocked the world in 1997 with an outbreak in Hong Kong that had a high fatality rate. At that time, it was believed that influenza viruses needed a mammalian intermediary host to jump to humans. However, H5N1’s direct transmission from birds to humans defied this assumption.
Virologists like Gavin Smith, who leads Duke-NUS’ Emerging Infectious Diseases Programme, noted that the virus’s ability to directly infect humans without an intermediary host was unprecedented. The outbreak in Hong Kong was contained through drastic measures, including the culling of 1.6 million chickens and a seven-week closure of live poultry markets. Yet, H5N1 continued to evolve and spread, eventually establishing itself in Europe and Africa.
A Complex Path Ahead
The current strain of H5N1 poses a significant threat due to its adaptability. Influenza viruses are highly mutable, with their segmented RNA genomes allowing for frequent reassortment. This process can lead to new virus variants with potentially alarming characteristics. The H5N1 virus has already shown its ability to adapt to new hosts, including cows, and its rapid spread through the food production system increases the risk of further mutations and outbreaks.
Ooi Eng Eong, a clinician-scientist with Duke-NUS, explained the complexity of influenza’s genetic makeup and its potential for rapid evolution. As H5N1 continues to spread, the virus’s potential to acquire mutations that enhance its ability to infect humans remains a pressing concern.
Preventive Measures and Future Risks
In light of these developments, experts are calling for increased surveillance and preventive measures. Reducing the virus’s presence through culling infected animals and vaccinating high-risk populations is crucial. Additionally, updating pandemic vaccine strains and enhancing biosecurity in food production systems can help mitigate the risk of a larger outbreak.
Renzo Guinto, an associate professor of Global and Planetary Health, sees an opportunity to transform food systems in response to these outbreaks. “We have a chance to make our food systems more sustainable, resilient, and accessible,” he noted.
As H5N1 continues to challenge scientists and public health officials, understanding its evolution and spread will be critical in preventing future pandemics. The current outbreak serves as a stark reminder of the delicate balance between humans, animals, and viruses, and the need for vigilant monitoring and rapid response to emerging threats.
Conclusion
The spread of H5N1 to dairy cows and its ongoing evolution across species underscores the urgent need for a comprehensive approach to managing infectious diseases. As scientists and public health officials work to understand and control this outbreak, the world watches closely, aware that the next pandemic could be just around the corner.