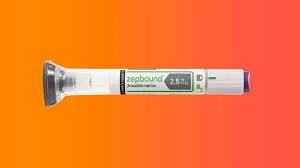
The U.S. Food and Drug Administration has given its approval for Zepbound (tirzepatide) injection, a new medication intended for chronic weight management in adults dealing with obesity (body mass index of 30 kg/m2 or greater) or overweight (body mass index of 27 kg/m2 or greater) accompanied by at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol). The medication is recommended for use alongside a reduced-calorie diet and increased physical activity. Tirzepatide, the active component in Zepbound, is already sanctioned under the name Mounjaro for improving blood sugar levels in adults with type 2 diabetes mellitus, in conjunction with diet and exercise.
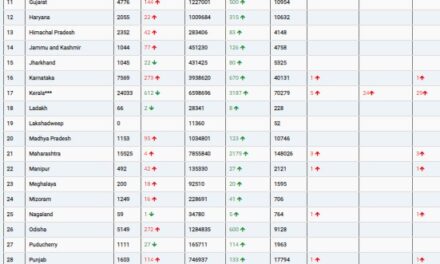
John Sharretts, M.D., director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research, emphasizes the importance of this approval in addressing the rising rates of obesity and overweight in the United States, as these conditions are linked to major causes of death such as heart disease, stroke, and diabetes.
Approximately 70% of American adults grapple with obesity or overweight, with a substantial portion facing weight-related health concerns. Studies have shown that losing 5% to 10% of body weight through diet and exercise can mitigate the risk of cardiovascular disease in adults dealing with obesity or overweight.
Zepbound functions by activating receptors of hormones from the intestine (glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)), effectively curbing appetite and food intake. Administered through weekly injections under the skin, the dosage must be gradually increased over four to 20 weeks to reach target dosages of 5 mg, 10 mg, or 15 mg once weekly, with the maximum dosage set at 15 mg once weekly.
The medication’s efficacy in chronic weight management, including weight reduction and maintenance, was established through rigorous trials involving adults with obesity or overweight and at least one weight-related condition. In these trials, patients receiving Zepbound experienced statistically significant reductions in body weight compared to those receiving a placebo, with a greater proportion achieving at least a 5% weight reduction.
Notably, Zepbound comes with potential side effects such as nausea, diarrhea, vomiting, constipation, abdominal discomfort, injection site reactions, fatigue, allergic reactions, burping, hair loss, and gastroesophageal reflux disease. It has also been associated with thyroid C-cell tumors in rats, and caution is advised in patients with a history of medullary thyroid cancer or Multiple Endocrine Neoplasia syndrome type 2.
The medication has not been studied in patients with a history of pancreatitis or severe gastrointestinal disease and should not be used in combination with Mounjaro or a GLP-1 receptor agonist. The safety and effectiveness of coadministration with other weight management medications have not been established.
Patients with a history of severe allergic reactions to tirzepatide or any of its ingredients, as well as those with conditions like pancreatitis, gallbladder problems, hypoglycemia, acute kidney injury, diabetic retinopathy, or a predisposition to suicidal behavior, should exercise caution and discuss potential risks with their healthcare providers.
Zepbound received Priority Review and Fast Track designations for this indication, and the FDA granted approval to Eli Lilly and Co.