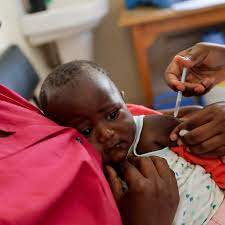
Côte d’Ivoire, West Africa – The Serum Institute of India (SII), in partnership with the University of Oxford, has officially rolled out its new high efficacy malaria vaccine, R21/Matrix-M, making Côte d’Ivoire the first country to begin administration. This significant milestone was reached on Monday, following the World Health Organization’s (WHO) approval of the vaccine last year.
Developed to combat the pervasive mosquito-borne disease, R21/Matrix-M has undergone rigorous clinical trials and regulatory assessments, demonstrating high effectiveness and affordability. As a low-dose vaccine, it can be manufactured rapidly and at scale, which is crucial for addressing the malaria burden across Africa.
“Reducing the malaria burden is finally within sight,” stated SII CEO Adar Poonawalla. “This launch marks a monumental milestone after years of collaboration with our partners at Oxford and Novavax.” He emphasized SII’s commitment to making essential disease prevention accessible to all, pledging to produce 100 million doses of R21 annually.
In preparation for this roll-out, SII has already manufactured 25 million doses and plans to offer the vaccine at an impressive price of under $4 per dose. This approach aims to ensure that the R21/Matrix-M vaccine is available to countries most in need, potentially transforming malaria control strategies across the continent.
Professor Adrian Hill, Director of the Jenner Institute at Oxford University, highlighted the importance of this development: “The R21/Matrix-M vaccine represents a new era in malaria control interventions, and we hope it will soon be available to all African nations interested in its use.”
The vaccine utilizes Novavax’s Matrix-M adjuvant technology and was granted WHO prequalification status in December 2023. Trials indicated it is well-tolerated, with injection site pain and fever being the most common adverse events reported.
Despite significant progress in reducing malaria-related deaths in Côte d’Ivoire—from 3,222 in 2017 to 1,316 in 2020—the disease continues to claim four lives daily, primarily among young children, and remains the leading cause of medical consultations in the country.
Côte d’Ivoire has received 656,600 doses of the vaccine, with plans to initially vaccinate 250,000 children aged 0 to 23 months across 16 regions. Other countries, including Ghana, Nigeria, Burkina Faso, and the Central African Republic, have also authorized the R21/Matrix-M vaccine.
R21 is the second malaria vaccine to be introduced in Sub-Saharan Africa, following RTS,S. The combination of malaria vaccines with existing prevention methods, such as bed nets, is expected to save tens of thousands of young lives annually. In total, 15 African countries are set to introduce malaria vaccines with Gavi support in 2024, aiming to reach approximately 6.6 million children by 2025.
Dr. Sania Nishtar, CEO of Gavi, the Vaccine Alliance, remarked, “Africa has borne the brunt of malaria for far too long, and Côte d’Ivoire has suffered more than most. With two safe and effective vaccines now available alongside other interventions, we can finally turn the tide against this killer disease.”
John Jacobs, CEO of Novavax, reinforced this sentiment, stating that the introduction of the R21/Matrix-M vaccine is a breakthrough in protecting vulnerable children against malaria, aligning with the mission to improve public health through innovative vaccines.