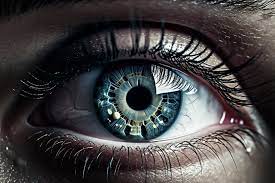
A recent study published in JAMA Ophthalmology has revealed a potential link between the use of semaglutide, a common diabetes medication, and an increased risk of nonarteritic anterior ischemic optic neuropathy (NAION), a serious cause of vision loss.
Researchers from China Medical University, led by Dr. Alan Y. Hsu, conducted a retrospective cohort study analyzing data from a global electronic health registry. The study, spanning from October 2019 to December 2023, compared 174,584 diabetes patients taking semaglutide with an equal number of patients taking other diabetes medications, such as metformin or sulfonylureas.
The findings indicated that semaglutide use was associated with a significantly increased risk of NAION. Specifically, the study reported a hazard ratio (HR) of 2.22, with a 95% confidence interval (CI) of 1.37-3.60, suggesting a more than doubled risk of developing NAION.
Interestingly, the increased risk was not observed during the first year of treatment. However, the risk became more pronounced at the 2-year (HR, 2.39; 95% CI, 1.37-4.18), 3-year (HR, 2.44; 95% CI, 1.44-4.12), and 4-year (HR, 2.05; 95% CI, 1.26-3.34) timepoints.
Subgroup analyses further highlighted that women, patients aged 40-64 years, White patients, and those with concurrent hypertensive diseases were at a significantly higher risk.
“Among patients with diabetes, an elevated risk of NAION was associated with semaglutide use compared with non–GLP-1 agents,” the authors concluded. They emphasized the importance of understanding the incidence and contributing factors of NAION to improve patient care.
An accompanying editorial suggested that future studies might benefit from comparing semaglutide to other second-line diabetes medications, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors, to mitigate potential biases.
Limitations:
The study’s retrospective design prevents the establishment of a direct causal link between semaglutide and NAION. The use of diagnostic codes may have introduced misclassification bias, and the longitudinal analysis might have been affected by loss to follow-up and limitations in representativeness.
Disclosures:
The study did not receive specific funding, and the authors reported no conflicts of interest.
Disclaimer: This news article is based on the provided study information and should not be interpreted as medical advice. Patients taking semaglutide should consult their healthcare providers for personalized guidance and to discuss any concerns regarding potential risks. Further research is necessary to confirm these findings and establish definitive causality.