University of Pittsburgh Study Finds Innovative Approach to TB Vaccine Offers Enhanced Safety and Efficacy
A groundbreaking study from the University of Pittsburgh has unveiled a self-destructing tuberculosis (TB) vaccine that provides stronger protection against the deadly disease in macaque monkeys. Published in Nature Microbiology, the research marks a significant milestone in the search for a more effective and safer vaccination strategy for TB, a disease recently named the deadliest of 2024 by the World Health Organization.
The innovative vaccine, administered intravenously, incorporates built-in safety mechanisms to prevent accidental self-infection by weakened mycobacteria. This novel approach presents a significant breakthrough in overcoming one of the biggest challenges in TB vaccination — ensuring both safety and effectiveness.
“Although the idea of intravenous vaccination with a live vaccine may sound concerning, our previous studies with non-human primates showed it was highly effective,” said Dr. JoAnne Flynn, distinguished professor and chair of microbiology and molecular genetics at the University of Pittsburgh. “In this new study, we focused on making the vaccine safer by engineering a strain of mycobacteria that self-destructs once administered. To our surprise, it was just as effective, if not more so, than traditional TB vaccines, providing sterilizing immunity to nearly all the animals.”
Traditional TB vaccines, like the Bacillus Calmette-Guérin (BCG) vaccine, provide partial protection, especially in young children, but fail to protect adults and do not prevent infection in immunocompromised individuals. The new self-destructing vaccine offers an alternative that could overcome these limitations, offering enhanced protection without the risk of vaccine-derived infection, even for those with weakened immune systems.
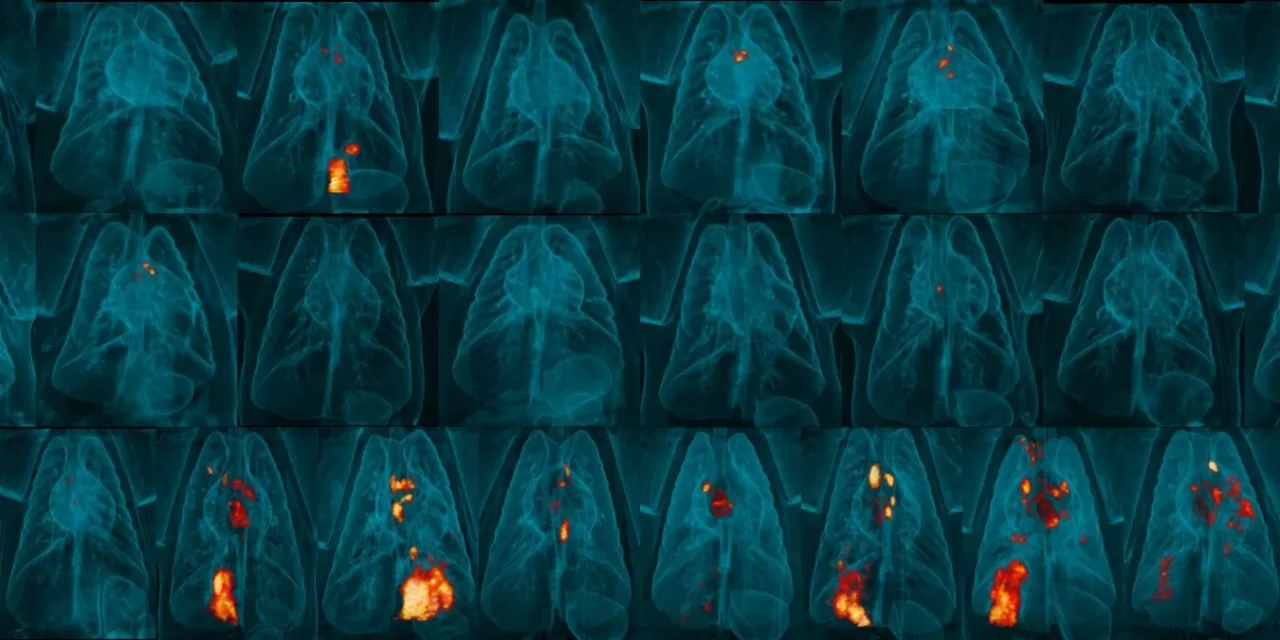
In the study, the researchers combined the safety of the vaccine’s self-destructing mechanism with its efficacy, resulting in an improved immune response in macaque monkeys. The updated vaccine delivered intravenously was found to be more effective than the standard BCG vaccine. Notably, none of the monkeys that received the new vaccine showed detectable lung inflammation after being exposed to live Mycobacterium tuberculosis, the bacteria responsible for TB.
The research also demonstrated that six out of eight monkeys vaccinated with the updated BCG strain showed no traces of recoverable live M. tuberculosis, compared to only two out of eight monkeys that received the standard intravenous BCG vaccine. This highlights the enhanced protective capabilities of the self-destructing vaccine.
Flynn’s previous research, in collaboration with the National Institutes of Health (NIH), demonstrated that intravenous BCG vaccination could significantly reduce bacterial load in the lungs of macaques. However, safety concerns over intravenous administration remained a challenge. This new study addresses those concerns by introducing a “kill switch” mechanism to the BCG vaccine, allowing it to safely dissolve upon exposure to the antibiotic doxycycline or when chronic treatment is stopped. This development ensures a faster elimination of the vaccine, even in immunocompromised animals.
Although clinical testing in humans remains a significant hurdle, researchers are optimistic about the future of this self-destructing BCG vaccine. “We hope that this ‘kill switch’ BCG strain could limit safety concerns over intravenous vaccine administration and provide a safer, more effective vaccination option for individuals who are immunocompromised,” Dr. Flynn added.
As TB continues to pose a global public health challenge, the hope is that this innovative vaccine could revolutionize the fight against one of the world’s most persistent and deadly infectious diseases.
For more information, refer to the study, A BCG kill switch strain protects against Mycobacterium tuberculosis in mice and non-human primates with improved safety and immunogenicity, published in Nature Microbiology (2025).
Journal Information: Nature Microbiology
DOI: 10.1038/s41564-024-01895-4