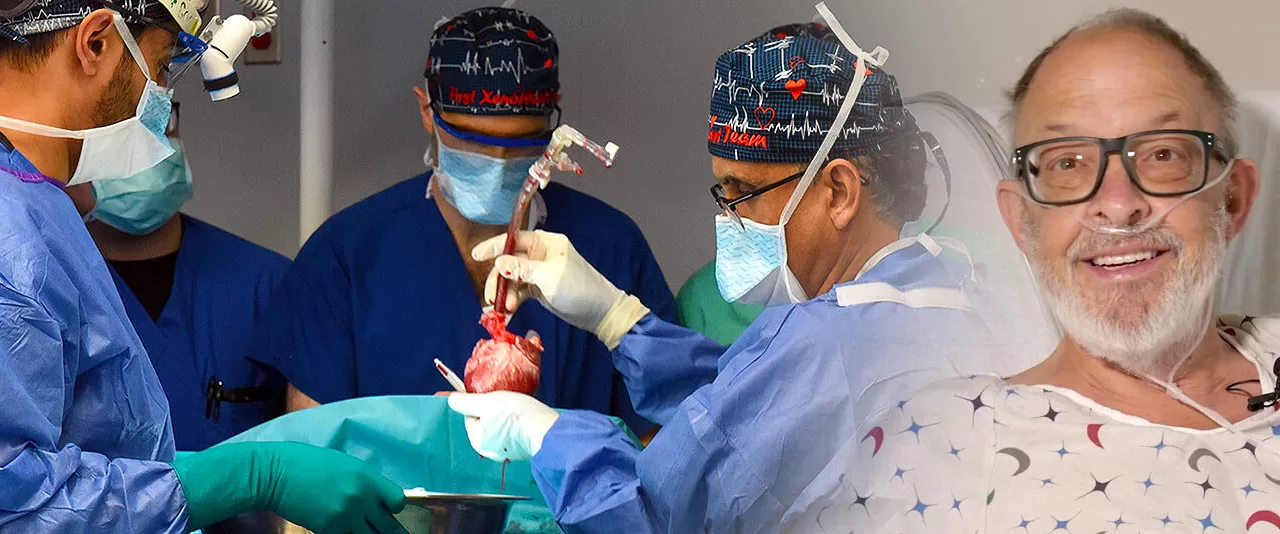
January 9, 2025 – In the realm of xenotransplantation, significant strides are being made as surgeons and scientists continue to push the boundaries of organ transplantation. A groundbreaking study from the University of Maryland School of Medicine provides important insights into the second patient in the world to undergo a genetically modified pig heart transplant. The patient, Lawrence Faucette, 58, received the pig heart at the University of Maryland Medical Center (UMMC) in 2023, aiming to treat his end-stage heart failure.
Mr. Faucette’s transplant, which was part of a pioneering study, was initially promising, with the 10 gene-edited pig heart demonstrating excellent systolic and diastolic function during the early weeks post-surgery. However, after 40 days, the heart began to fail due to rejection, and Mr. Faucette chose to forgo further treatment. His decision to participate in the procedure has provided critical insights into the future of xenotransplantation.
The report detailing these findings, published in Nature Medicine, highlights the lessons learned from Mr. Faucette’s experience. The most significant takeaway is the discovery of early signs of immune rejection within two weeks of the transplant, which points to ongoing challenges that need to be overcome for xenotransplantation to become a viable long-term solution for organ shortages.
Dr. Bartley P. Griffith, who performed the surgery and is a lead author of the study, explained, “We have taken another important step forward in the quest to address the global shortage of donor hearts. Thanks to the bravery of Mr. Faucette and his family, we now have a clearer understanding of the modifications we can make moving forward to achieve longer-term success.”
Mr. Faucette’s condition, which left him ineligible for a traditional human heart transplant, made the xenotransplant the only viable option. Despite the eventual failure of the transplant, the surgical team remains hopeful that these insights will pave the way for overcoming challenges such as antibody-mediated rejection in future procedures.
Dr. Muhammad M. Mohiuddin, another co-lead author of the study, emphasized the invaluable contributions of Mr. Faucette and his family, whose participation has propelled the field forward. “Their sacrifice yielded crucial scientific insights into how we and others should proceed to prevent graft failure in future transplants.”
The study identifies a key challenge: the surge in anti-pig antibodies, even in patients with low pre-existing levels. This immune response led to damage to the transplanted heart and, ultimately, graft failure. To address this, the team suggests that future transplants may require more aggressive strategies to suppress these antibodies and reduce the risk of rejection.
Despite these setbacks, the researchers remain optimistic about xenotransplantation’s potential as a long-term solution to the global organ shortage. Dr. Mark T. Gladwin, Dean of the University of Maryland School of Medicine, likened the current challenges in xenotransplantation to the early days of solid organ transplantation, where overcoming immune rejection was a significant hurdle.
“The insights gained from Mr. Faucette’s journey, alongside the learnings from our first pig heart transplant recipient, are guiding us as we continue to work towards understanding how to prevent organ rejection despite aggressive immunosuppression,” Dr. Gladwin noted.
As the field of xenotransplantation moves closer to becoming a clinical reality, Dr. Christine Lau, co-author of the study, expressed gratitude for the support provided by United Therapeutics, a key partner in this groundbreaking work.
Bert W. O’Malley, President and CEO of UMMC, praised the contributions of Mr. Faucette and his family, stating, “Their bravery and gratitude have been an inspiration. Their sacrifices have helped illuminate the path forward for this transformative medical advancement.”
The study, titled Transplantation of a genetically modified porcine heart into a live human, offers crucial lessons for overcoming the remaining obstacles in xenotransplantation, and its findings will serve as a foundation for upcoming clinical trials.
For more information, read the full study in Nature Medicine here.