February 28, 2025
Researchers from the Max Planck Institute of Biochemistry, in collaboration with teams from Heidelberg and Yale Universities, have made a groundbreaking discovery in the life cycle of the human immunodeficiency virus (HIV). Their study, published in the journal Nature, uncovers the long-elusive function of a component known as “spacer peptide 2,” which plays a crucial role in transforming immature HIV-1 particles into infectious agents.
Unraveling the HIV Maturation Process
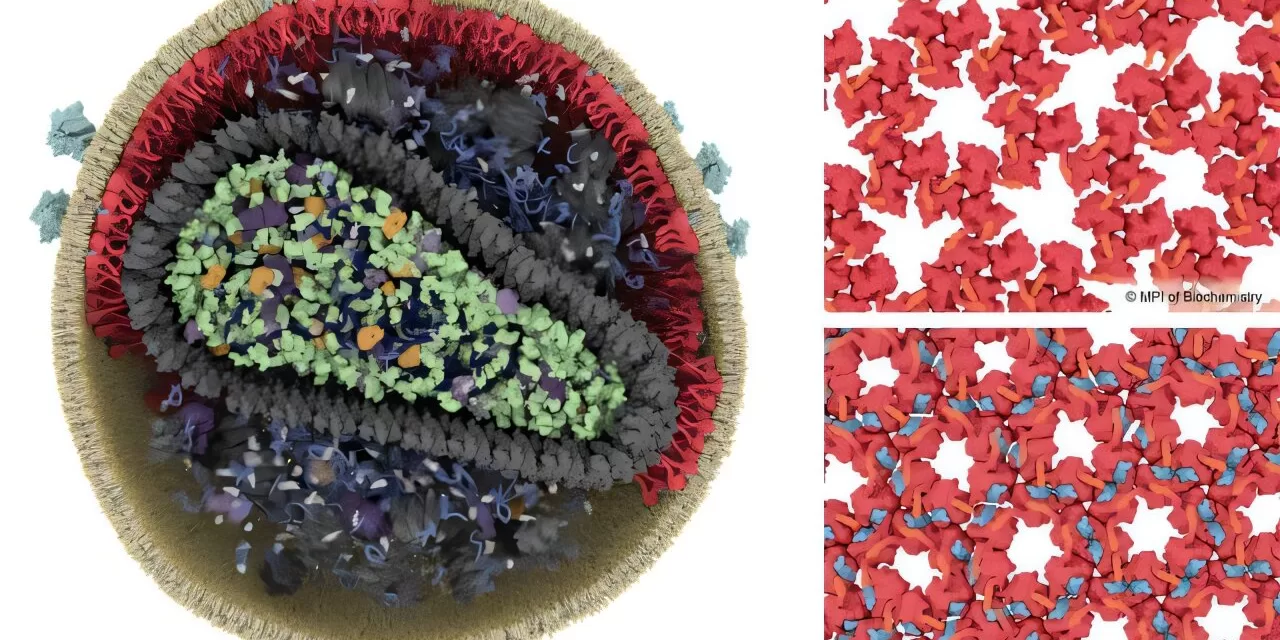
HIV-1, the primary virus responsible for AIDS, initially exists in an immature, non-infectious state after being released from an infected cell. The viral particle is primarily composed of around 2,000 copies of a rod-shaped protein called Gag. The maturation process, necessary for the virus to become infectious, involves the HIV-1 protease—a viral enzyme that cleaves Gag into six smaller proteins, including the capsid and matrix proteins. This cleavage prompts a significant structural reorganization of the virus.
While much research has focused on the rearrangement of the virus capsid, the outer protein shell (matrix) has remained less understood. However, a team led by John Briggs, a structural biologist and director at the Max Planck Institute of Biochemistry, has now shed light on how the matrix protein reorganizes during this crucial phase of HIV-1 maturation.
The Role of Spacer Peptide 2
Using advanced cryo-electron microscopes and computational image analysis, the researchers produced highly detailed 3D models of viral proteins. Their findings revealed that the structural transformation of the matrix is triggered by spacer peptide 2, which binds to the matrix protein and alters its packing arrangement.
“In our lab, we obtained the first structural data about the matrix in 2021, but we didn’t know what caused it to rearrange when the virus matures. In this new study, we produced much more detailed 3D views of the matrix layer, which was key to understanding what is going on,” said Briggs.
The study’s first authors, James Stacey and Dominik Hrebík, further explained the significance of their findings. “The mature virus matrix has a pocket, and we initially believed that a lipid from the viral membrane occupied this space. However, our research shows that spacer peptide 2 actually binds there, linking matrix proteins together in the mature virus,” said Stacey. Hrebík added, “This discovery highlights a previously unknown function of spacer peptide 2, suggesting potential avenues for therapeutic interventions.”
Implications for Future HIV Research
Understanding how spacer peptide 2 contributes to viral maturation opens new possibilities for targeted treatments. If researchers can develop molecules that disrupt this interaction, they may be able to hinder HIV-1’s ability to become infectious, paving the way for new antiretroviral therapies.
“HIV-1 is probably the most extensively studied virus, yet we continue to uncover vital aspects of its replication process that were previously unknown,” Briggs concluded.
Reference
James C. V. Stacey et al., “The conserved HIV-1 spacer peptide 2 triggers matrix lattice maturation,” Nature (2025). DOI: 10.1038/s41586-025-08624-9
Disclaimer: This article is based on scientific research and is intended for informational purposes only. It does not constitute medical advice or guidance for treatment. Readers should consult healthcare professionals for medical concerns related to HIV/AIDS.