December 31, 2024 – A groundbreaking study by scientists at Weill Cornell Medicine has revealed that the key to cellular aging may lie in the size of a tiny structure within our cells, known as the nucleolus. These findings, published in Nature Aging, uncover a biological “mortality timer” that could significantly impact the way we approach aging and age-related diseases.
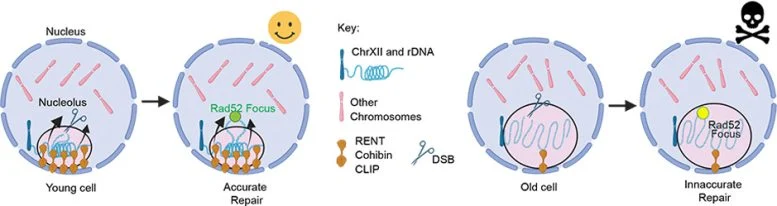
The nucleolus, a dense structure found within the cell’s nucleus, is responsible for housing ribosomal DNA (rDNA), which encodes the RNA portions of ribosomes—the cell’s protein-building machinery. As cells age, the nucleolus expands, leading to a series of events that ultimately result in cell death. In contrast, smaller nucleoli, akin to those seen in anti-aging strategies like calorie restriction, appear to slow the aging process and extend cell life.
Dr. Jessica Tyler, a professor at Weill Cornell Medicine, and her team studied this phenomenon using yeast, a model organism known for its simplicity yet surprising similarity to human cells. Their research suggests that maintaining a smaller nucleolus could delay the onset of aging, offering a potential avenue for future therapies aimed at extending human lifespan.
“Aging is the highest risk factor for diseases like cancer, cardiovascular disease, and neurodegenerative conditions,” explained Dr. Tyler. “Rather than treating each disease individually, our goal is to develop a therapeutic that can delay these diseases by targeting the underlying molecular causes, and it seems the nucleolus may hold the key.”
The Nucleolus: The Key to Cellular Youth?
The nucleolus plays a critical role in protecting the rDNA, which is one of the most fragile parts of our genome due to its repetitive nature. If the rDNA becomes damaged and isn’t repaired properly, it can cause chromosomal rearrangements that ultimately lead to cell death. As organisms age, including humans, the nucleolus tends to grow larger, which contributes to genomic instability and the decline of cellular function.
Interestingly, anti-aging interventions like calorie restriction have been shown to keep the nucleolus small, preserving its function. Dr. Tyler and her colleague, Dr. J. Ignacio Gutierrez, sought to isolate the nucleolus’s size as a factor independent of other anti-aging mechanisms to investigate its direct role in aging. They engineered yeast cells to control when the rDNA was anchored to the nuclear membrane, allowing them to manipulate the size of the nucleolus.
The results were striking: maintaining a small nucleolus delayed aging to a similar extent as calorie restriction, providing the first direct evidence of how nucleolar size could influence cellular lifespan.
A “Mortality Timer”
The team made another remarkable discovery: the growth of the nucleolus was not a gradual process over time. Instead, the nucleolus remained small for most of the yeast’s life, but once it reached a certain threshold size, it expanded rapidly. This sudden growth signaled the impending end of the cell’s life. After surpassing this threshold, cells could only survive for an average of five more cell divisions.
“We realized that the increase in nucleolar size wasn’t linear, which indicated something significant was happening,” Dr. Gutierrez said. “When the nucleolus crossed a specific size, it seemed to trigger the end of the cell’s lifespan.”
This sudden growth of the nucleolus serves as a “mortality timer,” signaling the final moments of a cell’s life. The study suggests that as the nucleolus enlarges, the rDNA becomes more unstable, and crucial proteins that are typically excluded from the nucleolus begin to enter, destabilizing the cell’s genetic material and leading to catastrophic DNA damage.
Dr. Tyler explained, “The nucleolus is supposed to separate biological reactions to make them more efficient. But as it grows, it becomes leaky, allowing harmful molecules to disrupt the fragile rDNA and triggering genomic instability.”
Implications for Longevity
These findings could pave the way for new anti-aging therapies that target the nucleolus to slow cellular aging. The researchers plan to explore the role of the nucleolus in human stem cells, which are critical for tissue regeneration. While stem cells initially have the ability to replace dead or damaged cells, they eventually stop dividing, contributing to aging and the decline of tissues. Understanding how nucleolar size influences stem cell longevity may lead to breakthroughs in extending their lifespan and, by extension, human health.
Dr. Gutierrez expressed excitement about the potential for these findings to be translated into human applications: “We’ve connected the structure of the nucleolus to the repair process in a way that seems to be conserved across species, from yeast to humans. This could be a powerful tool in extending healthy lifespans.”
With further research, these discoveries could lead to innovative strategies to delay aging and extend life, offering new hope for combating age-related diseases.
Reference:
Gutierrez, J. I., & Tyler, J. K. (2024). A mortality timer based on nucleolar size triggers nucleolar integrity loss and catastrophic genomic instability. Nature Aging. DOI: 10.1038/s43587-024-00754-5.