July 24, 2024
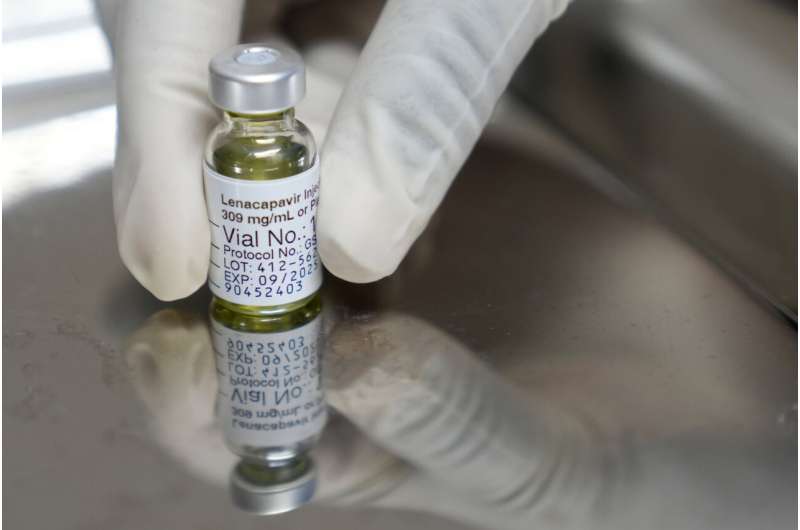
Munich, Germany — In a groundbreaking development for HIV prevention, a study has revealed that a twice-yearly injection offers complete protection against the virus. The study, involving approximately 5,000 women and girls in South Africa and Uganda, reported no new HIV infections among those who received the injections. This dramatic finding marks a significant advancement in the fight against HIV and AIDS.
The injections, developed by U.S. pharmaceutical company Gilead and marketed under the brand name Sunlenca, were administered every six months as part of a clinical trial. In contrast, participants who took daily prevention pills, such as Truvada or Descovy, saw about 2% of them contracting HIV from infected partners. The results were published on Wednesday in the New England Journal of Medicine and presented at an AIDS conference in Munich.
Salim Abdool Karim, director of the AIDS research center in Durban, South Africa, described the findings as “stunning,” highlighting the unprecedented level of protection provided by Sunlenca. Although Gilead’s injection is already approved for HIV treatment in several countries, its use for prevention remains pending regulatory review, particularly as testing in men continues.
The study’s early termination and subsequent offer of the injections to all participants underscore the promising nature of Sunlenca. Thandeka Nkosi of the Desmond Tutu Health Foundation noted that the prospect of a twice-yearly shot could revolutionize HIV prevention by addressing adherence issues often associated with daily pills and reducing stigma.
Despite the enthusiasm surrounding Sunlenca, concerns persist about its affordability, especially for those in low-income countries where the epidemic is most severe. Gilead has indicated it will explore a “voluntary licensing program” to allow select generic producers to manufacture the drug. However, the lack of a clear pricing strategy remains a critical issue. The current cost of HIV treatments in the U.S. exceeds $40,000 annually, a figure that is prohibitive for many in developing nations.
Winnie Byanyima, executive director of the U.N. AIDS agency, has urged Gilead to collaborate with U.N.-backed programs to facilitate broader access through generic versions. Byanyima emphasized the importance of making Sunlenca affordable for those in greatest need, such as women and girls facing domestic violence and men who are criminalized for same-sex relationships.
Doctors Without Borders has echoed these concerns, stressing that making Sunlenca widely available could significantly impact the global epidemic. As HIV prevention strategies evolve, the need for affordable, effective solutions remains paramount.
Gilead’s Dr. Jared Baeten has promised ongoing discussions with generics manufacturers to expedite the availability of affordable options. The introduction of Sunlenca represents a pivotal moment in HIV prevention, potentially mirroring the earlier revolutionary impact of antiretroviral drugs that transformed HIV from a fatal diagnosis into a manageable condition.
As the global community awaits further developments, the promising results of Sunlenca inject new hope into the ongoing battle against HIV/AIDS.