A groundbreaking artificial intelligence-powered tool, MISO (Multi-modal Spatial Omics), has been developed by researchers at the Perelman School of Medicine at the University of Pennsylvania to revolutionize the detection of cell-level characteristics in cancer. The tool offers unprecedented capabilities in analyzing tissue samples as small as 400 square micrometers—approximately the width of five human hairs—unlocking a wealth of information previously inaccessible.
Published in Nature Methods on January 15, 2025, MISO has proven instrumental in identifying crucial aspects of various cancers by analyzing medical imaging data derived from patient tissue donations. This next-gen AI tool has already made significant strides in enhancing our understanding of bladder, gastric, and colorectal cancers, as well as non-cancerous brain tissues.
Key discoveries made possible by MISO include:
- Bladder Cancer: Identifying specialized cells linked to better responses to immunotherapy through the detection of tertiary lymphoid structures.
- Gastric Cancer: Distinguishing cancerous cells from the surrounding mucosa within tissue samples.
- Colorectal Cancer: Pinpointing distinct subclasses of malignant cells within a single tumor, shedding light on tumor complexity.
These findings could lead to more targeted treatments, offering hope for improved patient outcomes and survival rates. Such precise analysis would be nearly impossible without a tool like MISO, which can analyze hundreds of thousands of cells in a single sample.
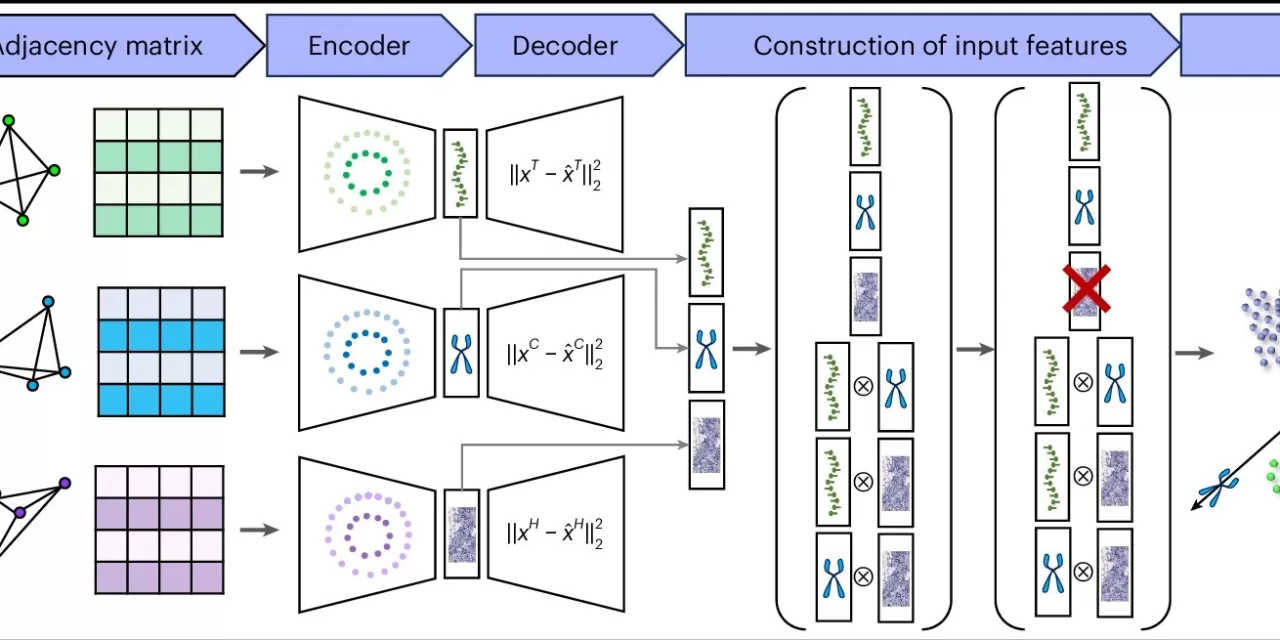
The power behind MISO lies in its ability to navigate the complex field of spatial multi-omics, where researchers study the physical layout of tissue and examine various molecular layers, such as gene expression, proteins, and metabolites. By analyzing a vast range of data points—20,000 to 30,000 data points per pixel in a single image—MISO provides a more holistic and insightful view of tissue compared to traditional imaging methods like MRI or CT scans, which only offer one data point per pixel.
According to Mingyao Li, Ph.D., the senior author of the study and a professor of Biostatistics and Digital Pathology, MISO addresses a significant challenge by enabling simultaneous analysis of all spatial -omics modalities and microscopic anatomy images. “MISO is the only method capable of handling such massive datasets, offering a comprehensive look at tissue that would be impossible to interpret with traditional methods,” Li said.
MISO represents the latest innovation in AI-driven imaging technologies, following Li’s previous work with iSTAR, a tool focused on genomics. While iSTAR helps sharpen images and generate spatial-omic data, MISO dives deeper, uncovering even the smallest biological markers like high-endothelial venules, specialized cells that help recruit immune cells to tissue.
Looking ahead, Li and her team plan to expand MISO’s capabilities to analyze multiple tissue samples simultaneously, vastly increasing its output. The AI tool’s adaptive learning system also allows it to incorporate new data, such as epigenetic marks, as it becomes available, offering the potential for even greater insights into cancer and cellular behavior.
For researchers and clinicians, MISO could be a game-changer in personalized medicine, offering more accurate diagnoses, more tailored therapies, and ultimately, better outcomes for patients battling cancer.
For more information, refer to the study Resolving Tissue Complexity by Multimodal Spatial Omics Modeling with MISO in Nature Methods (DOI: 10.1038/s41592-024-02574-2).