As individuals with congenital heart diseases experience longer lifespans, researchers are delving into the complexities of associated complications that arise with age. A team from the Children’s Hospital of Philadelphia (CHOP) has leveraged cutting-edge technologies to unravel the underlying biology of Fontan-associated liver disease (FALD), shedding light on its development and potential avenues for treatment.
Published in Science Translational Medicine, the findings offer unprecedented insights into the mechanisms driving FALD, providing a foundation for future therapeutic interventions.
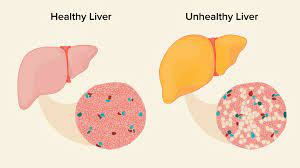
The Fontan operation stands as the standard treatment for single-ventricle congenital heart disease, redirecting blood flow from the inferior vena cava directly to the pulmonary arteries. While life-sustaining, this circulation method poses challenges, particularly the risk of venous congestion, potentially leading to FALD. With approximately 80,000 individuals worldwide having undergone the Fontan procedure, understanding and addressing FALD has become increasingly critical.
Dr. Jack Rychik, Director of the Fontan Rehabilitation, Wellness, Activity and Resilience Development (FORWARD) Program at CHOP, emphasized the study’s significance in enhancing patient care and outcomes. By unraveling the fundamental biology of FALD, researchers aim to pave the way for improved treatment strategies and enhanced quality of life for individuals born with single-ventricle congenital heart disease.
Through comprehensive analysis of human FALD tissue samples at a single-cell level, researchers generated the first complete atlas of RNA and epigenetic statuses. This revealed profound changes in specific liver cell types, particularly central hepatocytes, which exhibited significant metabolic reprogramming preceding disease onset. Furthermore, the study identified Activins A and B as potential therapeutic targets, shedding light on their involvement in fibrosis development associated with FALD.
Dr. Liming Pei, senior study author and associate professor at CHOP, underscored the importance of these findings in potentially blunting the cellular processes leading to liver fibrosis. The identified pathways hold promise for further investigation, offering potential avenues for the development of novel therapeutic options to mitigate FALD progression.
As research progresses, the aim is to translate these discoveries into tangible clinical benefits, ultimately improving the prognosis and quality of life for individuals living with congenital heart diseases and FALD.
The study signifies a significant step forward in the understanding and management of FALD, marking a crucial advancement in the field of congenital heart disease research.