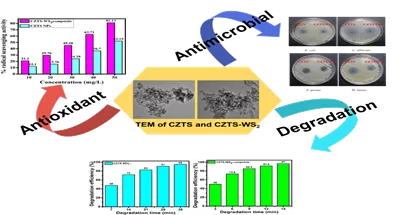
The periodic update of viruses contained in influenza vaccines is necessary for the vaccines to be effective due to the constant evolving nature of influenza viruses, including those circulating and infecting humans.
The WHO recommends that quadrivalent vaccines for use in the 2023-2024 northern hemisphere influenza season contain the following:
Egg-based vaccines
- an A/Victoria/4897/2022 (H1N1)pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus; and
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
Cell culture- or recombinant-based vaccines
- an A/Wisconsin/67/2022 (H1N1)pdm09-like virus;
- an A/Darwin/6/2021 (H3N2)-like virus;
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
WHO recommends that trivalent vaccines for use in the 2023-2024 influenza season in the northern hemisphere contain the following:
Egg-based vaccines
- an A/Victoria/4897/2022 (H1N1)pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus; and
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
Cell culture- or recombinant-based vaccines
- an A/Wisconsin/67/2022 (H1N1)pdm09-like vi;
- an A/Darwin/6/2021 (H3N2)-like virus; and
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus