Spinal muscular atrophy (SMA), a devastating neurodegenerative disorder that begins before birth, has seen a breakthrough in prenatal treatment. Scientists at St. Jude Children’s Research Hospital have successfully administered the oral drug risdiplam in utero, marking a significant milestone in SMA research.
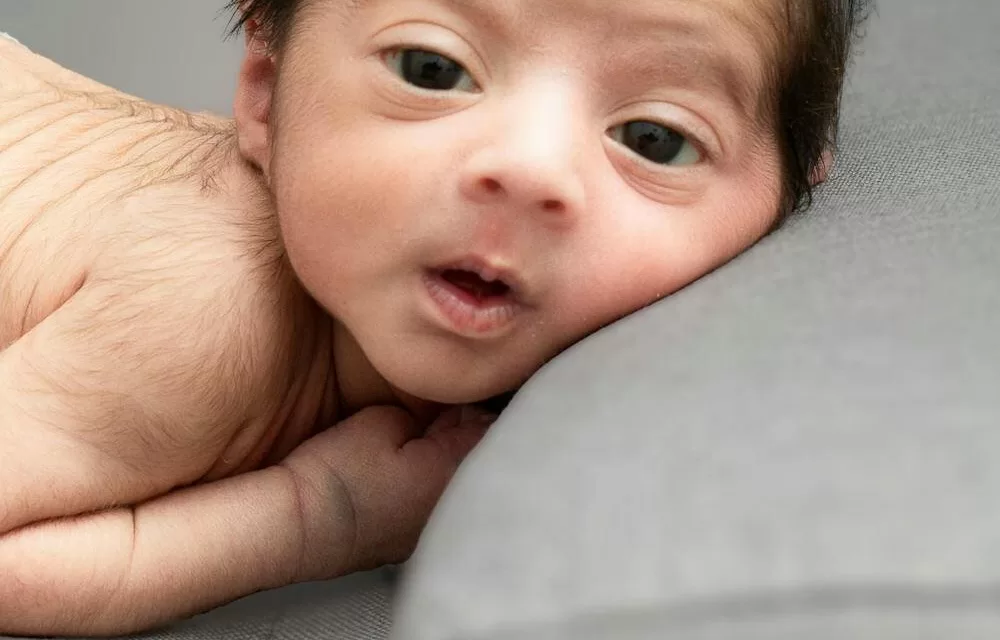
More than two years after birth, the treated child has exhibited no identifiable features of SMA. This promising outcome suggests that prenatal intervention could be a viable strategy for managing the disease. The findings were recently published in the New England Journal of Medicine.
A Groundbreaking Approach to SMA Treatment
Dr. Richard Finkel, the study’s corresponding author and director of the St. Jude Center for Experimental Neurotherapeutics, emphasized the study’s focus on feasibility, safety, and tolerability. “Our primary objectives were feasibility, safety, and tolerability, so we’re very pleased to see that the parent and child are doing well,” Finkel stated.
SMA is caused by a deficiency of survival motor neuron protein and affects approximately one in every 11,000 births in the United States. The most severe form, SMA type 1 (SMA-1), leads to progressive muscle weakness and, if left untreated, results in early mortality. Current treatments have improved survival and motor function when administered shortly after birth, but they do not constitute a cure.
Case Study: Prenatal Risdiplam Treatment
Recognizing the critical need for early intervention, St. Jude researchers initiated a novel clinical protocol under the Pediatric Translational Neuroscience Initiative to study the effects of prenatal risdiplam administration. The study focused on a single patient whose parents were known SMA genetic variant carriers. Their previous child had suffered from SMA-1 before modern treatments were available and had passed away at 16 months.
Genetic testing via amniocentesis confirmed that the fetus had no copies of the survival motor neuron gene, making it highly likely the infant would be born with SMA-1. As a result, risdiplam was administered to the expectant mother during the final six weeks of pregnancy.
Safe and Encouraging Outcomes Spur Future Research
Following birth, the infant was diagnosed with three developmental abnormalities—ventricular septal defect (which later resolved), optic nerve hypoplasia, and brainstem asymmetry—likely arising early in fetal development before exposure to risdiplam. Despite these challenges, the child, now two-and-a-half years old, has shown no clinical signs of SMA.
“During the course of the assessment, we really have seen no indication of any signs of SMA,” Finkel reported. These results underscore the safety and feasibility of the approach and support further investigation into prenatal therapy as a potential game-changer for SMA management.
Future Implications
This pioneering study offers hope for families affected by SMA and sets the stage for more extensive clinical trials. As researchers continue to explore prenatal treatment options, the possibility of mitigating SMA before birth may become a reality.
More Information: Richard S. Finkel et al., Risdiplam for Prenatal Therapy of Spinal Muscular Atrophy, New England Journal of Medicine (2025). DOI: 10.1056/NEJMc2300802
Journal Information: New England Journal of Medicine
Disclaimer: This article summarizes recent research findings and should not be interpreted as medical advice. Patients and families should consult healthcare professionals for medical guidance and treatment options.