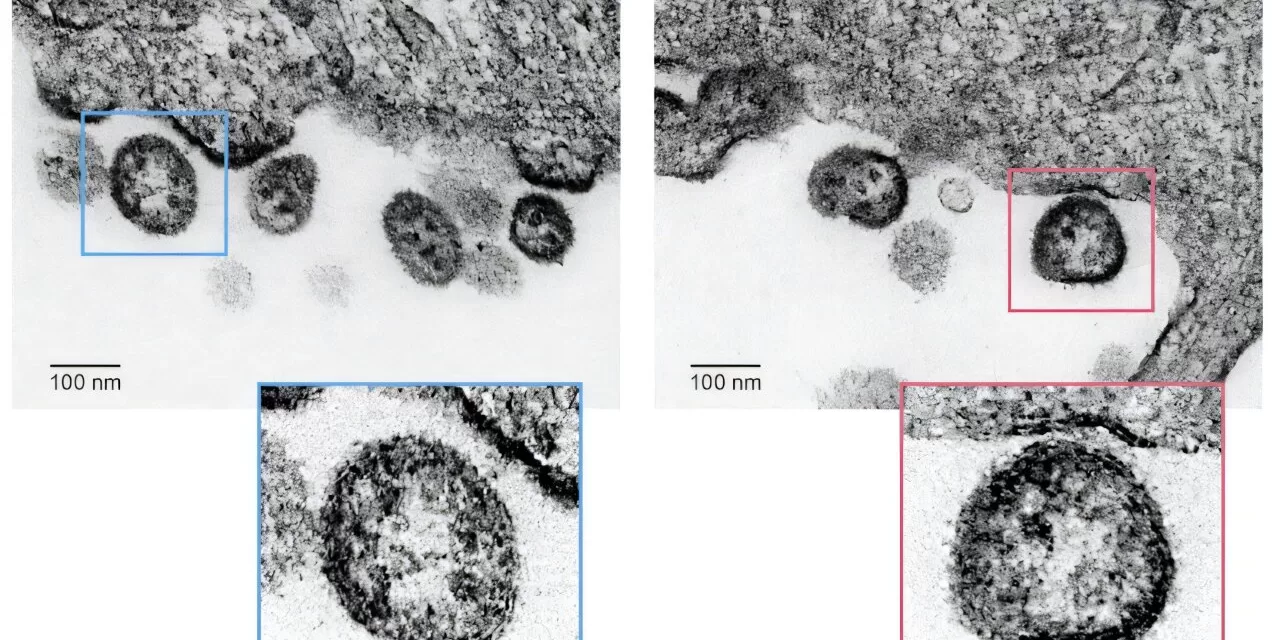
A groundbreaking live-attenuated vaccine candidate for Lassa virus has shown promising results in protecting guinea pigs from an otherwise lethal dose of the virus. Researchers from Texas Biomedical Research Institute (Texas Biomed), The Scripps Research Institute, and the National Institute of Allergy and Infectious Diseases (NIAID) recently reported the success of their vaccine candidate in npj Vaccines, offering hope for a future solution to the deadly disease.
Lassa virus causes tens of thousands of cases of Lassa fever annually, with an estimated fatality rate of 15-20%. Spread primarily by rodents, the virus can lead to severe symptoms including fever, organ failure, and bleeding. Despite its high morbidity and mortality, there is currently no approved vaccine or cure for Lassa fever, making it a priority for global vaccine research.
For over a decade, Dr. Luis Martínez-Sobrido from Texas Biomed and Dr. Juan Carlos de la Torre from Scripps Research have been working together to develop a vaccine against this deadly virus. They focused on creating a live-attenuated vaccine, a type of vaccine that uses a weakened version of the virus to stimulate an immune response. This approach has been successful for other vaccines, such as those for measles, mumps, and rubella.
A key challenge in developing a live-attenuated vaccine is ensuring the virus cannot revert to its original, dangerous form or combine with circulating strains to cause disease. To address this, the researchers employed two distinct methods to alter the virus’s genome. Dr. Martínez-Sobrido’s team used codon deoptimization to reduce the virus’s ability to bind to host cells, while Dr. de la Torre’s team modified the virus’s RNA to further reduce its replication capabilities. These combined edits produced a virus that was both safe and capable of eliciting a strong immune response.
“This is unbreakable attenuation,” said Dr. Martínez-Sobrido. “By modifying both segments of the virus, even if it were to mix with a wild virus, it would remain attenuated and unable to cause disease.”
The researchers tested the vaccine in 50 guinea pigs, exposing vaccinated animals to a lethal dose of Lassa virus. All vaccinated guinea pigs remained healthy, with no adverse side effects or signs of infection, demonstrating 100% protection.
“We couldn’t have hoped for better results,” Dr. Martínez-Sobrido said, emphasizing the vaccine’s potential for real-world use.
The team now plans to expand testing to nonhuman primates, which are considered the gold standard for assessing the safety and efficacy of vaccines before human trials. If successful, the vaccine could become a critical tool in preventing Lassa fever, a disease that remains a significant public health threat in Western Africa.
This research was supported in part by NIAID and conducted in collaboration with NIAID’s Integrated Research Facility at Fort Detrick. The study’s findings have generated excitement within the scientific community, offering new hope for the development of a much-needed vaccine against Lassa virus.
For more details, refer to the study: A Lassa virus live attenuated vaccine candidate that is safe and efficacious in guinea pigs, npj Vaccines (2024). DOI: 10.1038/s41541-024-01012-w.