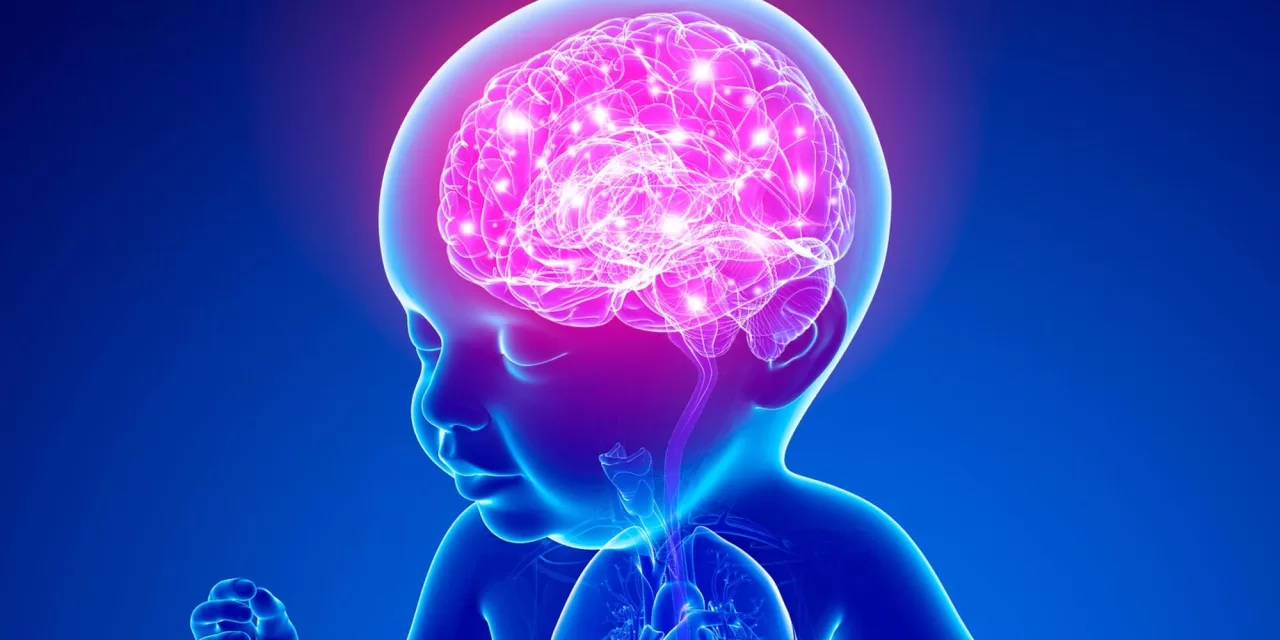
Brain cancer stands as the second-leading cause of death among children in the developed world. For those fortunate enough to survive, the long-term impacts of current treatments—such as chemotherapy and radiation—often diminish their quality of life, particularly among infants and young children. However, new groundbreaking research emerging from Emory University and the QIMR Berghofer Medical Research Institute in Queensland, Australia, suggests a potential therapy could revolutionize the way childhood brain cancers are treated, particularly those that are resistant to conventional methods.
The study, published in Nature Communications, presents the experimental drug CT-179, which has shown promise in preclinical mouse models. This novel therapy is designed to target a specific subset of tumor cells responsible for the recurrence and therapy resistance in pediatric brain cancer. With the potential to penetrate deep into the tumor and selectively disrupt cancer stem cells, CT-179 could offer a new hope for treating some of the most aggressive and persistent types of brain cancers in children, such as medulloblastoma, glioblastoma (GBM), and diffuse intrinsic pontine glioma (DIPG).
Professor Timothy Gershon, a pediatric neurologist at Children’s Healthcare of Atlanta and a leading researcher in this study, hailed the findings as a significant leap in understanding the biological mechanisms behind tumor recurrence. “Current treatments, including radiation and chemotherapy, often fail to eradicate cancer stem cells, which can regenerate the tumor. Our research shows that CT-179 specifically targets these cancer stem cells, making it an ideal candidate to complement existing therapies,” Gershon said.
This innovative therapy works by targeting OLIG2, a protein known to be a critical marker in cancer stem cells and a driver of tumor growth. By targeting this protein, CT-179 could prevent the recurrence of brain tumors after initial treatment.
Professor Bryan Day, who leads the Sid Faithfull Brain Cancer Laboratory at QIMR Berghofer, expressed his excitement about the drug’s potential. “This is a breakthrough for pediatric brain cancer research. It offers a solution to a problem that has been a long-standing challenge—tumor recurrence after conventional therapies,” Day noted. “The drug crosses the blood-brain barrier and enhances the efficacy of radiation therapy, potentially improving survival rates and quality of life for young patients.”
The findings of this international study complement similar work by the University of Toronto’s Professor Peter Dirks and his team, who also identified OLIG2 as a key protein in medulloblastoma and other pediatric brain tumors. Their research further confirmed that inhibiting OLIG2 could disrupt cancer stem cells and prevent tumor growth.
This collaboration, which spanned multiple countries including Canada, the U.S., Australia, and Sweden, marks a significant step forward in the fight against pediatric brain cancer. The next phase of research will focus on clinical trials, with the hope that CT-179 can soon be tested in human patients.
While the results are promising, researchers caution that much work remains before this therapy becomes a standard treatment. Clinical trials will be necessary to determine the safety and efficacy of CT-179 in children with brain cancer.
Disclaimer: The information in this article is based on preclinical research and is not yet verified in clinical trials. The drug CT-179 is still in the early stages of testing and has not been approved for use in human patients. Further studies are needed to confirm its safety and effectiveness in treating pediatric brain cancer.