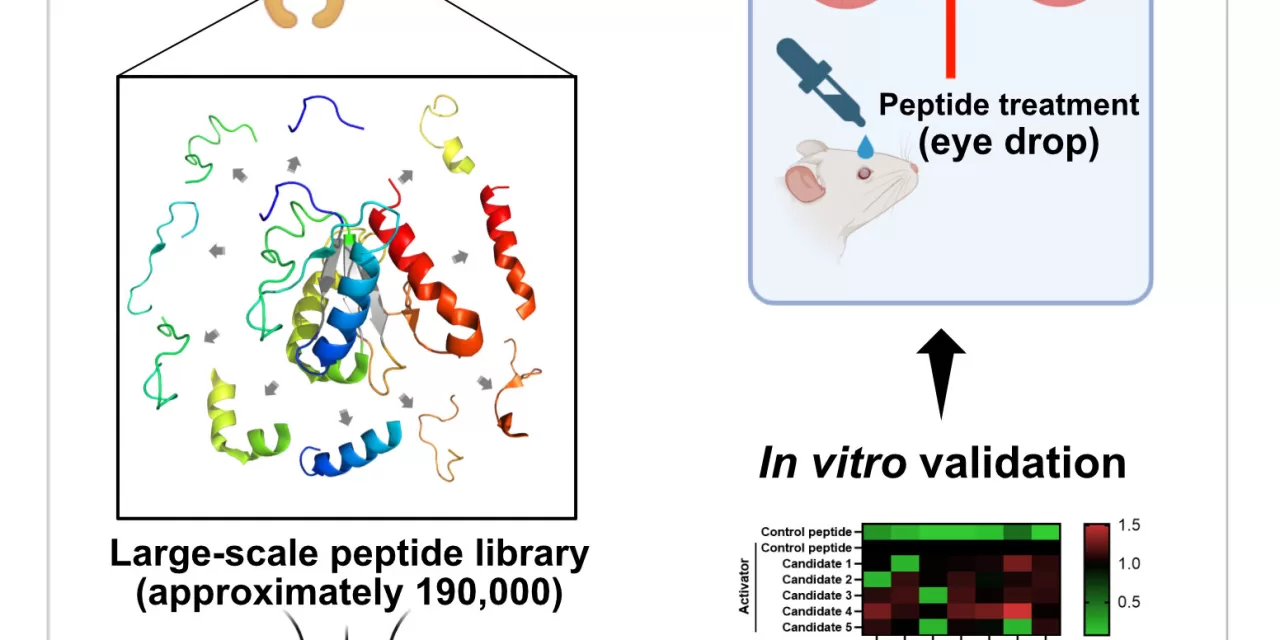
In a groundbreaking development, researchers have unveiled a potential new treatment for dry age-related macular degeneration (AMD) using peptide-based eye drops, marking a significant step forward in ophthalmic care. This new treatment, which can be administered as eye drops, offers a promising alternative to the current FDA-approved injectable therapies for dry AMD, providing hope for millions of patients worldwide.
The research team, led by Dr. Moon-Hyeong Seo at the Korea Institute of Science and Technology (KIST), has developed this innovative therapeutic agent after addressing the significant challenges posed by conventional treatments. As of 2023, the only FDA-approved treatments for dry AMD are two injectable drugs, both of which are associated with complications from intravitreal injections and limited effectiveness in restoring vision.
Eye drops, in contrast, are the most preferred method of drug delivery in ophthalmology due to their convenience and non-invasive nature. However, targeting the retina, located in the posterior segment of the eye, with eye drops has been a major hurdle in eye care. Dr. Seo’s team sought to overcome this obstacle by focusing on the inflammatory signaling pathway of Toll-like receptors (TLRs), which play a crucial role in the progression of AMD.
Through the extraction of peptide sequences from thousands of proteins similar to TLR signaling proteins, the researchers developed a vast library of over 190,000 potential peptide drug candidates. By leveraging advanced technology to screen these peptides for their ability to specifically bind to TLR signaling proteins, the team identified several peptides capable of inhibiting harmful interactions within this pathway.
The team then tested these peptides in an animal model by administering them as eye drops to mice with induced dry AMD. The results were promising, with the treated mice showing significant retinal protection and reduced degeneration, bringing their retinal health back to levels comparable to that of normal, healthy mice. This evidence suggests that peptide-based eye drops could potentially replace injectable therapies, offering a more convenient and effective treatment option.
Beyond the therapeutic potential, the non-invasive nature of the treatment could greatly improve patient compliance and satisfaction, while reducing the risks and costs associated with repeat injections. The development of this treatment also signals a shift toward safer, more accessible treatments for AMD and other ophthalmic conditions.
Dr. Seo, who leads the Natural Product Drug Development Center at KIST, emphasized the global significance of this innovation. “Our center, which was established with a focus on mission-driven research, is dedicated to developing drugs targeting aging-related diseases, including cancer and ophthalmic conditions. We are excited to collaborate with domestic and international pharmaceutical companies to advance global clinical trials for this groundbreaking therapy,” he said.
This peptide-based treatment represents a significant leap forward in the field of ophthalmology and could be a game-changer in how dry AMD is treated in the future. With the potential to reduce the burden of invasive treatments, this innovation is expected to transform treatment accessibility, providing new hope for individuals suffering from age-related vision loss.
For further details, the full study can be found in the journal Advanced Science.
Source: Yun Lim et al, “Massively Parallel Screening of Toll/Interleukin‐1 Receptor (TIR)‐Derived Peptides Reveals Multiple Toll‐Like Receptors (TLRs)‐Targeting Immunomodulatory Peptides,” Advanced Science, 2024. DOI: 10.1002/advs.202406018