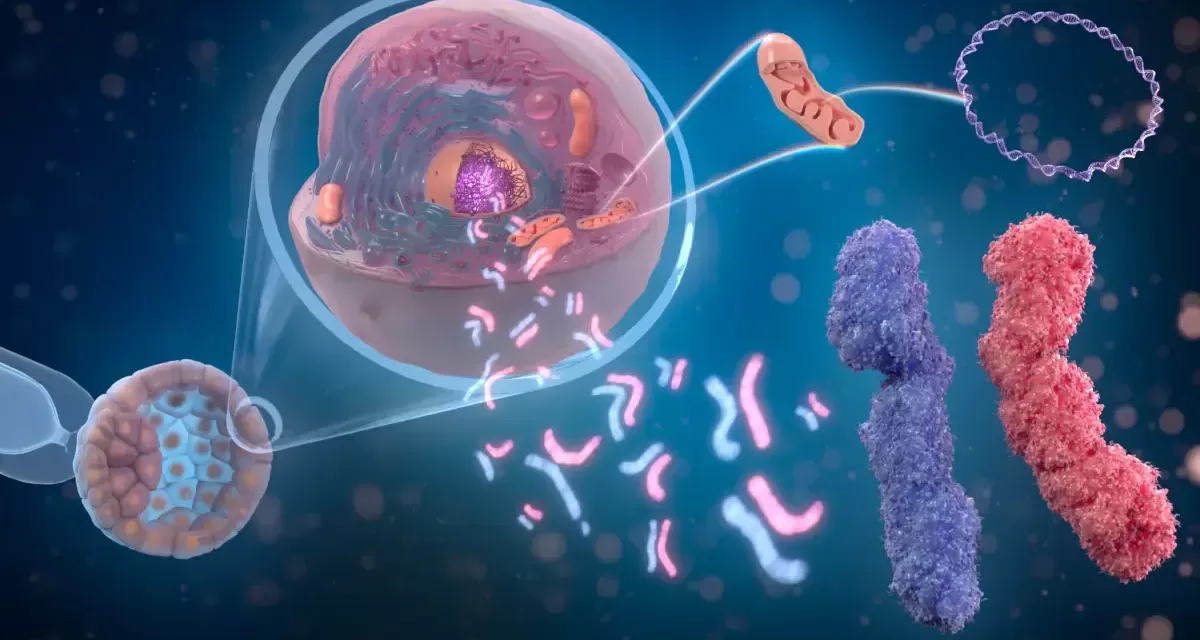
A groundbreaking inhalable gene therapy that could significantly improve lung disease in people with cystic fibrosis (CF) is currently being tested in human trials across the UK and Europe. This innovative treatment aims to benefit all CF patients, regardless of their genetic mutation type.
Cystic fibrosis is a life-threatening inherited condition caused by defects in the CFTR gene, leading to thick and sticky mucus accumulation in the lungs and digestive system. This results in chronic lung infections and a gradual decline in breathing ability. While CFTR modulator medicines have provided hope for many, they are ineffective for approximately 10–15% of patients due to their specific genetic mutations.
The new lentiviral vector-based gene therapy, known as BI 3720931, works by delivering a functional copy of the CFTR gene directly into airway epithelial cells. By doing so, it aims to enhance lung function and decrease the frequency of exacerbations, particularly for those who cannot benefit from existing CF therapies.
LENTICLAIR 1 Trial: A Step Toward a Breakthrough
The LENTICLAIR 1 trial, conducted by biopharmaceutical company Boehringer Ingelheim in collaboration with the UK Respiratory Gene Therapy Consortium (GTC) and OXB (formerly Oxford Biomedica), is assessing the safety, tolerability, and effectiveness of this novel treatment. Approximately 36 adult patients with CF are participating in the trial at specialized centers in the UK, France, Italy, the Netherlands, and Spain.
Professor Eric Alton, a leading figure in respiratory gene therapy and the head of the UK CF Gene Therapy Consortium, emphasized the significance of this milestone:
“After 24 years of dedicated research and collaboration, we have finally reached a crucial stage in our quest to provide an effective gene therapy for CF patients. This treatment holds the potential to bring long-term CFTR function improvement and even disease modification, making a meaningful difference in patients’ lives.”
Professor Jane Davies, UK Lead Investigator for the trial, echoed his sentiments:
“The advent of CFTR modulators has been transformative, but those unable to benefit from them urgently need alternative solutions. This trial represents a pioneering step in gene therapy, with the potential for lasting CFTR expression.”
How the Trial Works
In the initial phase, different doses of the gene therapy will be administered to evaluate safety and determine the optimal dosage for the next stage. The second phase will involve a randomized, double-blind, placebo-controlled study to assess its clinical efficacy and safety.
After completing the 24-week trial, participants will be monitored in a long-term follow-up study, LENTICLAIR-ON, to evaluate sustained benefits and any potential need for re-dosing. The trial is expected to conclude in early 2027, with more details available on ClinicalTrials.gov (NCT06515002).
A Hopeful Future for CF Patients
With over 105,000 people worldwide affected by cystic fibrosis and more than 2,000 known mutations of the CFTR gene, this trial represents a significant step toward a more inclusive and effective treatment approach. Lentiviral vector gene therapy harnesses the ability of modified lentiviruses to deliver therapeutic genes into human cells, offering a promising avenue for long-term disease management.
Disclaimer:
This article is for informational purposes only and does not constitute medical advice. Clinical trials are ongoing, and the safety and efficacy of this therapy are still being evaluated. Individuals should consult healthcare professionals for guidance on available CF treatments.