February 19, 2025
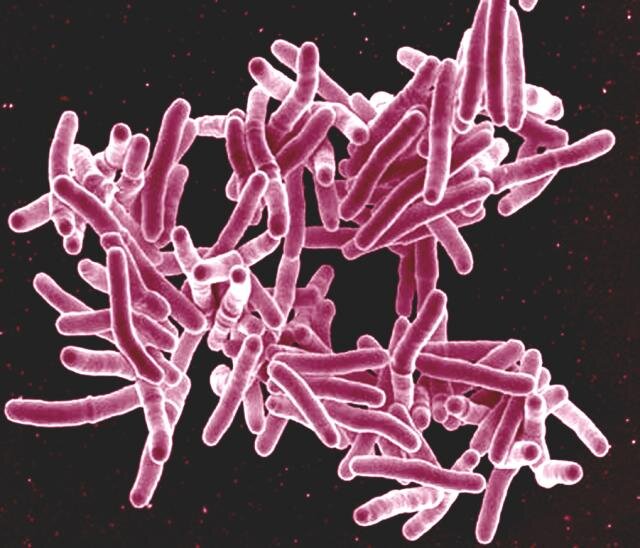
Every year, tuberculosis (TB) affects 10 million people worldwide, leading to approximately 1.5 million deaths. The disease, caused by Mycobacterium tuberculosis (Mtb), often requires prolonged antibiotic therapy, and the rise of drug-resistant TB strains has made the development of new treatments crucial. Recent advancements have highlighted the potential of BTZ-043, a novel antibiotic with significant promise in combating TB, particularly in hard-to-reach areas of the lungs.
Breakthrough Research on BTZ-043
Developed by researchers at the German Center for Infection Research (DZIF), in collaboration with the Institute for Infectious Diseases and Tropical Medicine at LMU University Hospital Munich and the Leibniz Institute for Natural Product Research and Infection Biology—Hans Knöll Institute (Leibniz-HKI) in Jena, BTZ-043 has demonstrated strong bactericidal activity in clinical trials. A study recently published in Nature Communications provides compelling evidence of its effectiveness.
Led by scientists from the University of Bayreuth, the Research Center Borstel—Leibniz Lung Center, and Johns Hopkins University, the study reveals that BTZ-043 efficiently penetrates TB lesions, accumulating in high concentrations where bacteria hide. This capability significantly enhances the drug’s effectiveness against TB, including drug-resistant strains.
Overcoming Granuloma Challenges
A hallmark of TB is the formation of granulomas—nodular structures in the lungs designed to contain Mtb bacteria. These structures include a fibrous outer layer, immune cells, and a necrotic core where bacteria persist. Due to poor blood supply in these necrotic areas, conventional antibiotics struggle to reach and eliminate the bacteria effectively.
Utilizing an advanced mouse model replicating human TB granulomas, the DZIF research team demonstrated BTZ-043’s ability to infiltrate necrotic lesions. High-resolution MALDI mass spectrometry confirmed that the antibiotic not only reached these difficult sites but also significantly reduced bacterial load within them.
Expert Insights
“Our study marks an important milestone in TB drug development, as it is the first to visualize the distribution of a clinical-stage TB drug within granulomas,” said Prof. Andreas Römpp of the University of Bayreuth, a lead researcher on the project.
Dr. Kerstin Walter from the Research Center Borstel added, “The ability of BTZ-043 to target these hard-to-reach lesions suggests a strong bactericidal effect, potentially improving TB therapy.”
Dr. Christoph Hölscher, coordinator of the DZIF research area “Tuberculosis,” highlighted the significance of the advanced mouse model, stating, “Unlike traditional models, this approach closely mimics human TB pathology, aiding the search for more effective treatments.”
Dr. Julia Dreisbach, Scientific Program Manager for BTZ-043 at the Tropical Institute Munich, emphasized the broader implications: “These findings offer hope for millions suffering from tuberculosis and suggest a future where even the most resilient TB lesions can be treated more effectively.”
Looking Ahead
The study underscores BTZ-043’s potential as a game-changing antibiotic in the fight against tuberculosis. As further research and clinical trials progress, the drug’s ability to enhance TB treatment outcomes may revolutionize current therapeutic strategies.
Disclaimer: The information in this article is based on ongoing research and should not be considered a substitute for professional medical advice. Further clinical trials are necessary to determine the full efficacy and safety of BTZ-043 for widespread use.