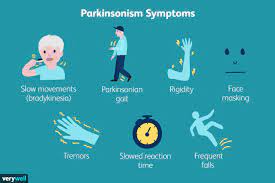
In a significant breakthrough for Parkinson’s disease research, a team of scientists has utilized computational models to unravel the molecular mechanisms underlying the accumulation of alpha-synuclein protein, a key culprit in the development of the disease. The study, published today as a Reviewed Preprint in eLife, offers important biophysical insights into the association of alpha-synuclein chains, providing a deeper understanding of Parkinson’s disease pathogenesis.
Led by Abdul Wasim, a PhD student at the Tata Institute of Fundamental Research, Hyderabad, India, and senior author Jagannath Mondal, Associate Professor at the Tata Institute of Fundamental Research, the research sheds light on the intricate dynamics of alpha-synuclein aggregation, a hallmark feature of Parkinson’s disease.
“Intrinsically disordered proteins (IDPs) like alpha-synuclein play pivotal roles in various diseases, including neurodegenerative disorders like Parkinson’s disease,” explains Wasim. “Our study aimed to elucidate the molecular mechanisms driving alpha-synuclein aggregation, which is essential for understanding disease progression.”
The researchers employed coarse-grained molecular dynamic simulations to study the collective interaction of alpha-synuclein chains within droplets under different environmental conditions. By simulating the aggregation of multiple alpha-synuclein proteins, the team uncovered key insights into the factors influencing protein aggregation.
Their findings revealed that environmental factors such as crowding and salt promote alpha-synuclein aggregation through distinct mechanisms. Crowder molecules, which create a highly crowded space for proteins, and salt both enhanced alpha-synuclein aggregation, albeit via different pathways. Importantly, the study elucidated the role of surface tension in protein aggregation, with salt increasing surface tension and crowder molecules having no surface tension effects.
Moreover, the researchers observed that alpha-synuclein proteins within dense liquid-liquid phase separated (LLPS) droplets exhibited an extended shape and consistent orientation, characteristic of the LLPS phenomenon. This suggests that alpha-synuclein IDPs display hallmarks of LLPS, a phenomenon implicated in neurodegenerative diseases.
Further analysis revealed key amino acid residues within alpha-synuclein that likely play a role in preventing aggregation, providing insights into the molecular basis of protein aggregation. However, the study acknowledges limitations, including the need for improved benchmarking of simulations against other methods.
“Our study underscores the importance of understanding the molecular basis of alpha-synuclein aggregation in Parkinson’s disease,” concludes Mondal. “By elucidating the mechanisms driving protein aggregation, we pave the way for targeted therapeutic interventions to combat Parkinson’s disease.”
The editors note the significance of the study’s findings in advancing our understanding of Parkinson’s disease pathogenesis and the potential for future therapeutic development. With further research, computational models hold promise for decoding the complex mechanisms underlying neurodegenerative disorders, offering hope for improved treatments and outcomes for patients.