A groundbreaking study published in Nature has revealed critical insights into why treatments for autoimmune and inflammatory diseases may inadvertently increase the risk of tuberculosis (TB). Researchers at Rockefeller University have discovered that a deficiency of a protein called tumor necrosis factor (TNF), which is often targeted by therapies for these conditions, plays a pivotal role in exacerbating TB risk.
TB: A Global Health Threat
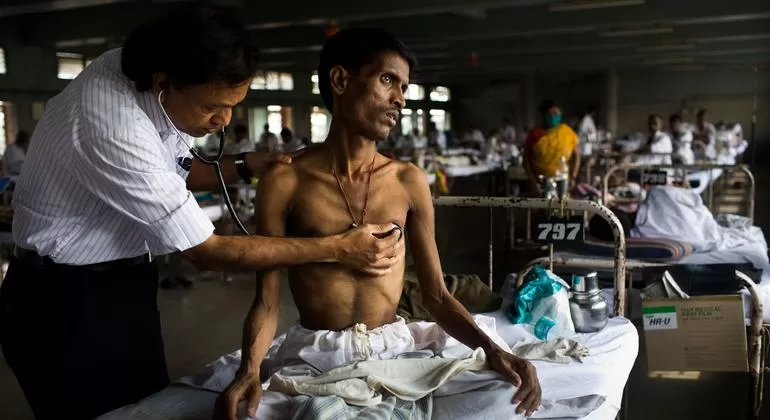
Tuberculosis remains the leading cause of death from infectious disease globally. Despite the widespread presence of Mycobacterium tuberculosis (Mtb), the bacterium responsible for TB, only about 5% of infected individuals succumb to the disease. TB primarily spreads through the air and typically affects the lungs, where it can cause severe respiratory issues.
The Role of TNF in TB Protection
For years, scientists have known that acquired deficiencies in TNF can elevate the risk of developing TB. However, the exact mechanisms remained elusive until now. The new study, led by Jean-Laurent Casanova and his team at Rockefeller University, has upended long-held beliefs about the immune system’s response to TB.
The researchers found that TNF plays a crucial role in protecting the lungs against TB infection, despite having a limited role in broader inflammation and immune responses. A lack of TNF disrupts a specific immune process within the lungs, rendering them vulnerable to severe TB infections.
Case Study: Unraveling a Genetic Mystery
The study focused on two individuals from Colombia, a 28-year-old woman and her 32-year-old cousin, who experienced recurrent TB infections. Despite responding well to initial anti-TB treatment, both patients became ill again within a year, raising concerns about an underlying issue.
To investigate, the research team conducted whole-exome sequencing on the two patients and analyzed the genetic profiles of their parents and relatives. They discovered that the two patients were the only members of their extended family with a mutation in the TNF gene, rendering the protein non-functional.
This genetic defect meant that the patients’ alveolar macrophages, the immune cells in their lungs responsible for engulfing and destroying Mtb, were unable to control the infection. The absence of functional TNF left their lungs vulnerable, leading to recurring and severe TB infections.
Implications for Autoimmune Disease Treatments
The study’s findings have significant implications for the treatment of autoimmune and inflammatory diseases, where TNF inhibitors are commonly used. These inhibitors, while effective in reducing inflammation, may inadvertently disable a key component of the immune system’s defense against TB.
The research solves a long-standing mystery about the increased susceptibility to TB among patients receiving TNF inhibitors. “Without TNF, a key part of the defense against TB is defunct,” Casanova and his team concluded, highlighting the importance of monitoring TB risk in patients undergoing such treatments.
Moving Forward
This discovery underscores the need for a more nuanced approach to treating autoimmune and inflammatory diseases, especially in regions where TB is prevalent. As researchers continue to explore the complex interplay between the immune system and infectious diseases, this study provides a vital piece of the puzzle in understanding how to protect vulnerable patients from TB while managing their underlying conditions.