A groundbreaking study published in Frontiers of Medicine has shed new light on the molecular pathology of esophageal squamous cell carcinoma (ESCC), a highly aggressive cancer with a poor prognosis. The research reveals an important role of the BOLA3 gene and its regulation by the heterogeneous nuclear ribonucleoprotein C (HNRNPC), offering fresh insights into how this pathway could be targeted for potential therapies.
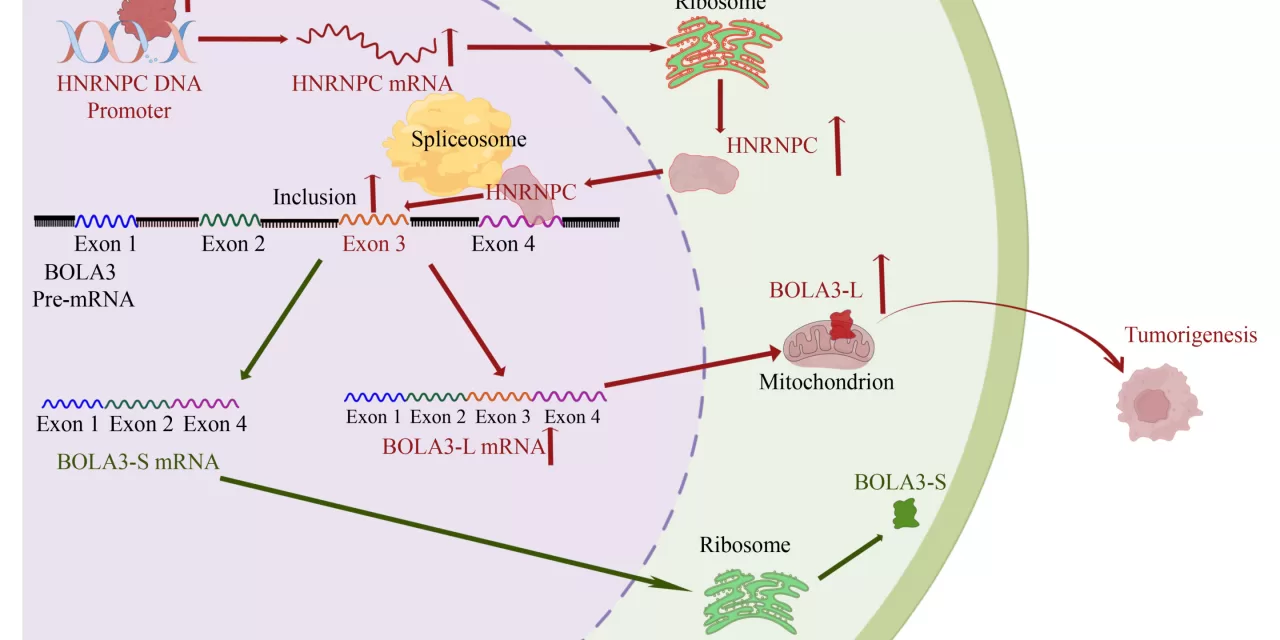
Esophageal squamous cell carcinoma, which arises from the cells lining the esophagus, is notorious for its rapid progression and lack of early symptoms, which often results in late-stage diagnoses. The study’s findings focus on a previously underexplored aspect of ESCC progression: the regulation of BOLA3 exon 3 inclusion, a process that is facilitated by the protein HNRNPC.
BOLA3, a protein involved in cellular functions such as the regulation of ribosome biogenesis, has been implicated in various cancers, but its precise role in ESCC had not been fully understood. The current study, led by Bo Tian and colleagues, has unveiled how HNRNPC enhances the inclusion of exon 3 in BOLA3, leading to an increase in BOLA3 protein production and subsequent promotion of ESCC cell proliferation, migration, and invasion.
Through experiments conducted on ESCC cell lines and clinical samples, the researchers found that HNRNPC was overexpressed in ESCC tissues compared to normal tissues. Notably, higher levels of HNRNPC were associated with poor tumor differentiation and advanced tumor stages, pointing to its potential role in increasing tumor aggressiveness.
The study’s authors demonstrated that HNRNPC directly interacts with the pre-mRNA of BOLA3, driving the inclusion of exon 3 and boosting the production of the BOLA3 protein. When BOLA3 exon 3 inclusion was enhanced, ESCC cells showed a significant increase in their malignant properties, such as colony formation, migration, and invasion. Conversely, reducing HNRNPC or BOLA3 expression inhibited these behaviors, highlighting the critical role of the HNRNPC-BOLA3 pathway in ESCC progression.
The researchers also explored the clinical implications of these findings by analyzing patient samples. They found a strong positive correlation between the expression levels of HNRNPC and BOLA3 in ESCC tissues. Notably, high levels of both proteins were associated with shorter overall survival, suggesting that HNRNPC and BOLA3 could serve as useful prognostic biomarkers.
In conclusion, the study presents a novel molecular mechanism involving HNRNPC and BOLA3 that accelerates ESCC progression. These findings open the door to targeted therapeutic strategies aimed at modulating the HNRNPC-BOLA3 axis, potentially offering new treatment options for patients with this aggressive cancer.
Further research is required to validate these findings and assess the clinical utility of HNRNPC and BOLA3 as both therapeutic targets and biomarkers in ESCC.
Disclaimer: The information provided in this article is based on the research study titled “Dysregulated inclusion of BOLA3 exon 3 promoted by HNRNPC accelerates the progression of esophageal squamous cell carcinoma” by Bo Tian et al. The conclusions drawn from the study are those of the authors and should be interpreted in the context of the current scientific literature. The research is ongoing, and further studies are necessary to confirm the therapeutic potential of the HNRNPC-BOLA3 axis.