August 9, 2024 — A groundbreaking study published in Nature Metabolism sheds light on how the brain regulates eating driven by necessity versus pleasure. Researchers have identified specific neural circuits in the mouse brain that not only promote hunger-driven feeding but also suppress eating for pleasure, potentially offering new avenues for combating obesity.
Eating habits are often driven by either physical hunger or the pleasure derived from consuming certain foods, even in the absence of hunger. While hunger-driven eating is essential for survival, pleasure-driven eating can contribute to obesity and related metabolic disorders. This new study highlights the role of neurons in the brain that regulate these two types of feeding behaviors differently.
Dr. Yong Xu, a co-corresponding author of the study and professor of pediatrics—nutrition at Baylor College of Medicine, emphasized the importance of balancing necessity and pleasure in eating. “Ideal feeding habits would balance eating for necessity and for pleasure, minimizing the latter,” he said. “In this study, we identified a group of neurons that regulates balanced feeding in the brain.”
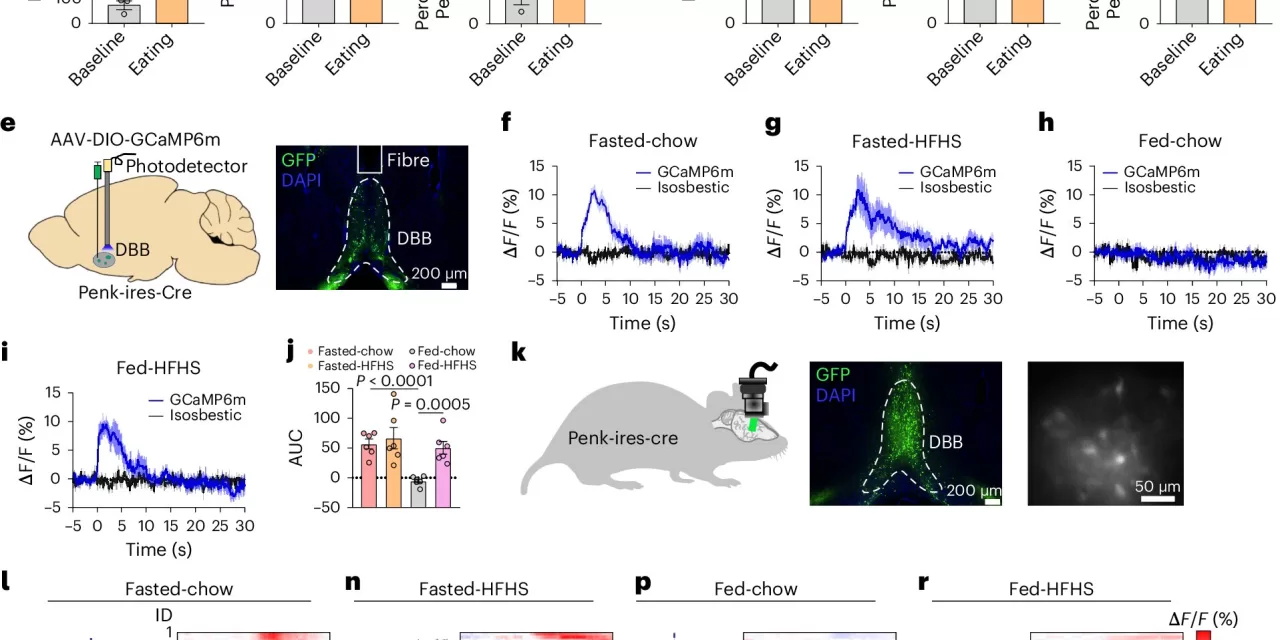
The study focuses on neurons marked by the GABAergic proenkephalin (Penk) marker, an endogenous opioid hormone previously known to play a role in feeding and body weight regulation. However, the specific contribution of these neurons to hunger- and pleasure-driven feeding had not been fully understood until now.
Xu and his team discovered that activating Penk neurons in the diagonal band of Broca (DBB) brain region in male mice enhanced hunger-driven feeding while simultaneously reducing pleasure-driven eating. This finding was unexpected, as previous research suggested that neurons affecting feeding behavior would impact both types similarly.
The researchers identified two distinct populations of DBB-Penk neurons projecting to different brain regions. One group projects to the paraventricular nucleus of the hypothalamus, which is activated during fasting and promotes hunger-driven feeding. The other projects to the lateral hypothalamus and is activated by high-fat, high-sugar (HFHS) foods, but interestingly, inhibits consumption of these reward-based foods.
“Mice with eliminated DBB-Penk neurons consumed less chow but increased their intake of HFHS foods, leading to faster development of obesity and metabolic issues,” Xu noted. “These findings suggest that obesity development may be linked to impaired function within these brain circuits.”
The study’s results offer promising insights into potential treatments for obesity and related metabolic disorders. Researchers plan to further investigate the molecular markers within these circuits to identify targets for human disease treatments.
For more details, refer to the study: Liu, H., et al. (2024). Distinct basal forebrain-originated neural circuits promote homeostatic feeding and suppress hedonic feeding in male mice. Nature Metabolism. DOI: 10.1038/s42255-024-01099-4.