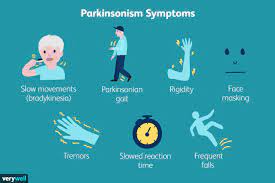
A breakthrough in the diagnosis of neurodegenerative disorders such as Parkinson’s disease (PD) may soon revolutionize early detection and treatment strategies. A recent study published in JAMA unveils the efficacy of a simple skin biopsy test in identifying an abnormal form of alpha-synuclein, a protein associated with synucleinopathies, with remarkable accuracy.
Led by Christopher Gibbons, MD, professor of neurology at Harvard Medical School, the research team conducted a blinded, multicenter trial to assess the effectiveness of the test in detecting phosphorylated alpha-synuclein (P-SYN) in individuals with PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure (PAF).
The study included 428 participants aged 40-99 years recruited from various neurology practices across the United States. Among them, 277 were diagnosed with synucleinopathies, while 120 served as a control group. Utilizing the Syn-One Test, researchers analyzed skin biopsies from each participant and observed a remarkable detection rate of P-SYN, reaching 95.5% accuracy overall.
Dr. Gibbons highlighted the significance of this advancement in neurological diagnostics, emphasizing the challenges faced by patients in obtaining accurate and timely diagnoses. “With a simple, minimally invasive skin biopsy test, this study demonstrated how we can more objectively identify the underlying pathology of synucleinopathies and offer better diagnostic answers and care for patients,” he stated.
Synucleinopathies, affecting an estimated 2.5 million people in the United States, present a pressing need for reliable diagnostic biomarkers. These progressive neurodegenerative diseases, characterized by the accumulation of P-SYN, often pose diagnostic dilemmas due to overlapping symptoms and variable prognoses.
Despite the promising results, the study authors acknowledge certain limitations, including the need for further validation in broader clinical settings and the potential for false positives in the control group. Additionally, the Syn-One Test is not currently FDA-approved for PD diagnosis but serves as a valuable tool for pathological assessment.
The development of effective diagnostic tools such as the Syn-One Test holds immense promise for early intervention and personalized treatment strategies in neurodegenerative disorders. With ongoing research and validation efforts, the potential impact of this innovative approach on patient care is substantial.
The study received funding from the National Institutes of Health, with Dr. Gibbons disclosing stock options in CND Life Sciences, the company behind the Syn-One Test. Further disclosures are detailed in the original article.