Alcohol use disorder (AUD), which affects approximately 400 million people globally, is a leading cause of serious health conditions such as cancer, liver disease, cardiovascular disease, and stroke. However, its impact extends beyond physical health, deeply affecting cognitive functions critical for learning, memory, and adaptability—core components of cognitive flexibility.
A recent study from the Texas A&M University College of Medicine, published in Science Advances, offers groundbreaking insights into how chronic alcohol use impairs cognitive flexibility, particularly by disrupting the brain’s cholinergic signaling pathways.
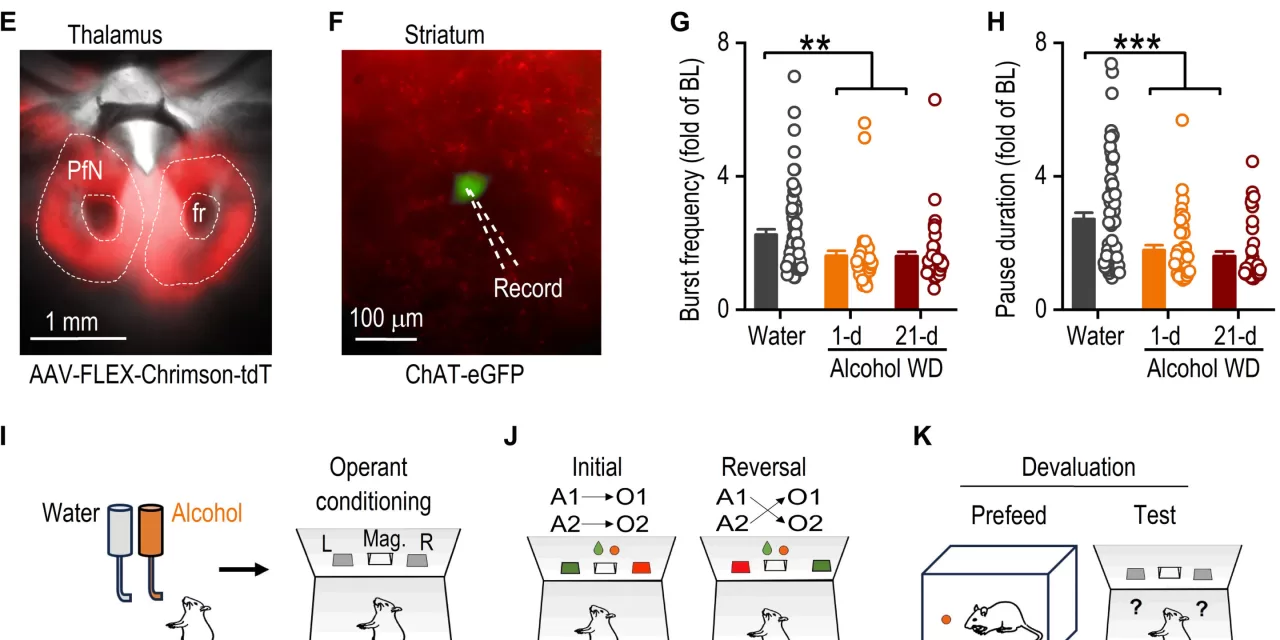
The research, led by Zhenbo Huang, Ph.D., in the lab of Jun Wang, MD, Ph.D., identifies a crucial role of cholinergic interneurons (CINs) in the brain’s striatum in regulating cognitive adaptability. CINs release acetylcholine, a key neurotransmitter involved in learning, reward-driven behaviors, and motivation by modulating dopamine signaling.
“Dopamine neurons drive the brain’s reward system, while CINs act as gatekeepers, filtering stimuli that activate these neurons,” explained Wang, an associate professor at the Texas A&M College of Medicine. “Alcohol disrupts the finely tuned balance of these systems, impairing the brain’s ability to adapt to changing circumstances.”
The research team used advanced techniques such as optogenetics—a method that utilizes light to control specific cells—and fiber photometry, which allows real-time tracking of acetylcholine release. These technologies enabled the scientists to observe how alcohol alters the firing patterns of CINs.
Under normal circumstances, CINs fire in a “burst-pause” pattern, where a rapid burst of activity is followed by a pause. This rhythm is crucial for behaviors such as learning and adapting to new situations, especially in tasks like reversal learning, where old habits must be unlearned and replaced with new behaviors. However, in models of chronic alcohol exposure, the CINs exhibited a disrupted firing pattern, with weaker and shorter pauses that impair the reversal learning process.
“Reversal learning is central to cognitive flexibility,” Wang noted. “It allows individuals to adjust their behavior when circumstances change—a process that is heavily reliant on acetylcholine signaling.”
The study revealed that the burst and pause phases of CIN activity have distinct roles. The “burst” phase, which promotes the release of acetylcholine, supports extinction learning, where old behaviors are suppressed. The “pause” phase, which reduces acetylcholine release, is crucial for reversal learning, facilitating the transition from outdated behaviors to new, adaptive ones.
This research provides important insights into the mechanisms behind alcohol-induced cognitive impairments, offering potential therapeutic targets for treating AUD-related cognitive dysfunction. Moreover, it raises the possibility that similar mechanisms may be at play in other brain conditions, such as neurodegenerative diseases and aging.
“The burst and pause dynamics of CINs are fundamental to behavioral adaptability,” Wang emphasized. “Our study paves the way for exploring how these processes might influence a broader range of conditions, beyond addiction.”
The research team continues to investigate how these mechanisms affect brain health and aims to develop innovative treatments for cognitive disorders.
This study marks a significant step forward in understanding the neurological impact of alcohol use disorder, opening new avenues for therapeutic interventions aimed at improving cognitive flexibility and overall brain function.
For more details, see: Zhenbo Huang et al, Dynamic responses of striatal cholinergic interneurons control behavioral flexibility, Science Advances (2024). DOI: 10.1126/sciadv.adn2446.