Researchers have developed a novel probiotic strain that demonstrates significantly enhanced effectiveness in combating giardiasis, a prevalent intestinal parasitic infection affecting both humans and animals, particularly dogs. The infection, caused by the protozoan Giardia intestinalis, leads to symptoms such as diarrhea, abdominal pain, nausea, and weight loss, and can even result in long-term complications like irritable bowel syndrome (IBS) and chronic fatigue.
The study, published in the journal Gut Microbes, highlights the growing concern of parasite resistance to conventional treatments like nitroimidazole. In response, scientists have been exploring the potential of Lactobacillus johnsonii CNCM I-4884, a probiotic strain patented by INRAE, MNHN, and EnvA in 2015. This bacterium exhibits significant anti-Giardia activity by hindering the parasite’s development within the intestine.
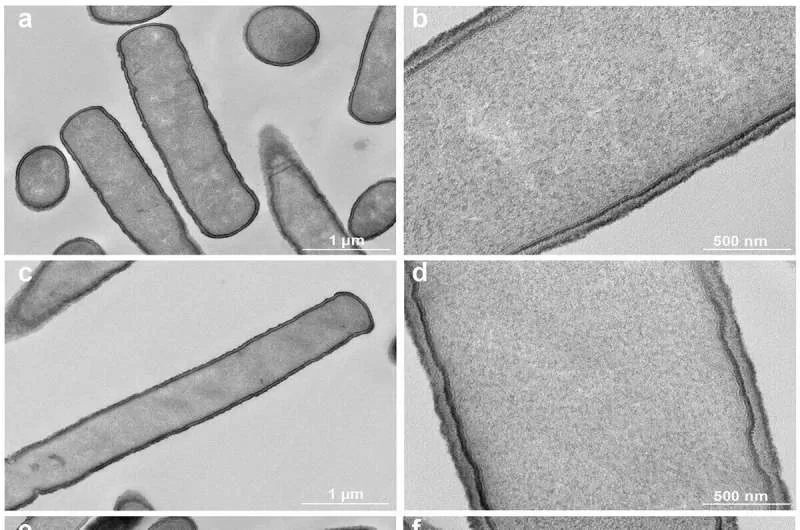
The researchers focused on enhancing the bile salt resistance of L. johnsonii CNCM I-4884, a crucial factor for its survival and efficacy in the digestive system. L. johnsonii naturally transforms conjugated bile salts, essential for G. intestinalis growth, into unconjugated bile salts, which are toxic to the parasite. This process is a natural part of digestion and also contributes to intestinal anti-inflammatory effects.
Through laboratory experiments involving exposure to increasing bile salt concentrations, the team identified a derivative strain with improved bile salt transformation activity and persistence during culture. In a murine model infected with G. intestinalis, this new strain demonstrated a 64.4% reduction in parasite load, compared to 48.8% for the original wild-type strain, representing a 15% increase in effectiveness.
“This improved derivative strain of L. johnsonii offers a significant advancement in the fight against giardiasis,” stated the researchers in their publication. “Its enhanced ability to resist bile salts and reduce parasite load holds promise for the development of more effective probiotic treatments.”
Currently, a probiotic drug based on L. johnsonii is in the final stages of clinical trials for dogs. The success of this study paves the way for the development of similar probiotic therapies for humans in the future.
More information: Anne-Sophie Boucard et al, Isolation of derivatives from the food-grade probiotic Lactobacillus johnsonii CNCM I-4884 with enhanced anti- Giardia activity, Gut Microbes (2025). DOI: 10.1080/19490976.2025.2474149
Disclaimer: This article is based on research findings and should not be taken as medical advice. Always consult with a healthcare professional for diagnosis and treatment of any medical condition. The probiotic drug mentioned is currently undergoing clinical trials for dogs and has not yet been approved for human use. Future research is needed to determine the safety and efficacy of this probiotic strain in humans.