February 20, 2025 – Researchers have uncovered new molecular mechanisms that contribute to insulin resistance, a key precursor to type 2 diabetes mellitus (DM2). The findings, published in Cell Communication and Signaling, offer fresh insights into how insulin resistance develops in skeletal muscle, the primary tissue affected by the condition, and suggest potential targets for future diabetes treatments.
Insulin resistance occurs when insulin is unable to effectively facilitate glucose uptake in tissues, leading to chronic hyperglycemia. Given that skeletal muscle consumes the most glucose in response to insulin, understanding its role in insulin resistance is crucial for advancing DM2 treatment strategies.
The study is led by Manuel Vázquez-Carrera from the University of Barcelona’s Faculty of Pharmacy and Food Sciences, in collaboration with researchers from the Institute of Biomedicine of the UB (IBUB), the Sant Joan de Déu Research Institute (IRSJD), and the Networking Biomedical Research Center’s Diabetes and Associated Metabolic Diseases area (CIBERDEM). Other contributors include Ricardo Rodríguez-Calvo (CIBERDEM and Universitat Rovira i Virgili), Antoni Camins (UBNeuro and CIBERNED), and Walter Wahli (University of Lausanne, Switzerland).
Investigating the Insulin Receptor’s Role
Diabetes is a major global health issue, affecting millions and leading to severe complications such as cardiovascular disease, kidney failure, and vision impairment. While previous studies have focused on the metabolic pathways activated by insulin, less attention has been paid to the function of the insulin receptor itself.
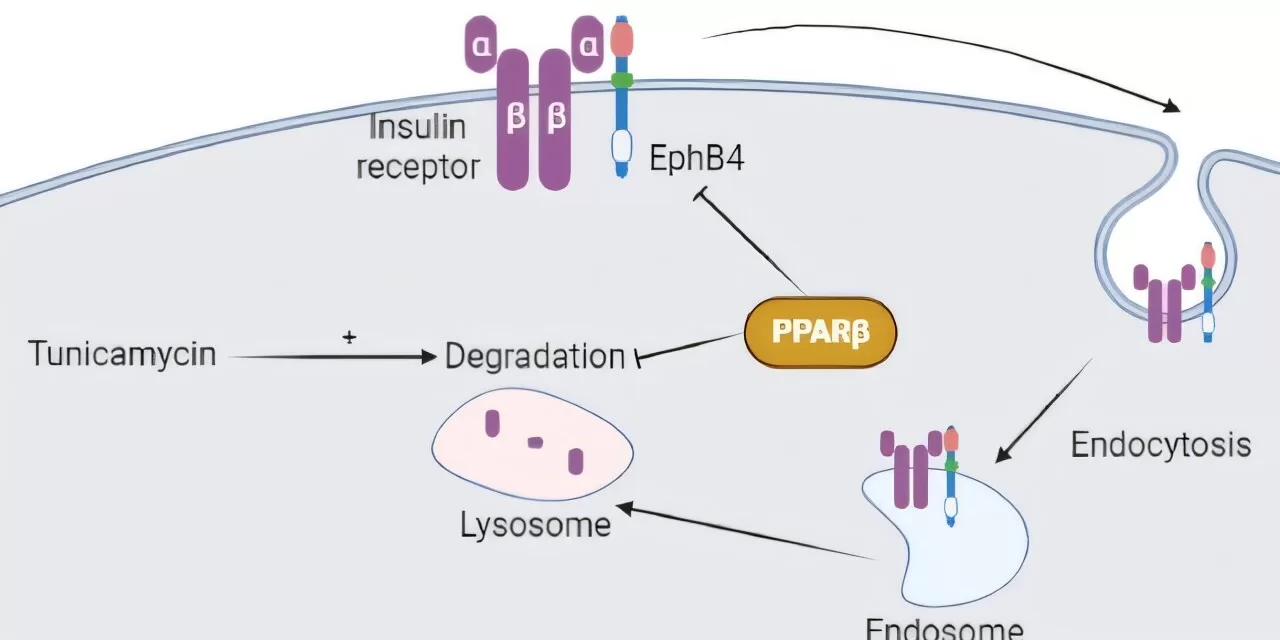
“The insulin signaling pathway begins when insulin binds to a receptor on insulin-responsive cells. This receptor consists of the α-subunit (InsRα) and the β-subunit (InsRβ), which plays a crucial role in initiating the glucose uptake process,” explained Professor Vázquez-Carrera.
The study explored whether peroxisome proliferator-activated receptor (PPAR) β/δ influences InsRβ levels in mouse muscle and cultured myotubes. Researchers found that mice lacking the PPARβ/δ gene had lower InsRβ protein levels in their skeletal muscle compared to normal mice. However, treatment with GW501516—a PPARβ/δ agonist—led to increased InsRβ protein levels in mouse muscle.
Potential Therapeutic Implications
The study also demonstrated that the reduction of InsRβ levels due to endoplasmic reticulum stress, a process linked to insulin resistance, could be partially reversed with the PPARβ/δ agonist. This compound was found to reduce both reticulum stress and lysosomal activity, which is responsible for degrading InsRβ protein.
Moreover, the researchers discovered that levels of ephrin receptor tyrosine kinase B4 (EphB4), a protein that binds to InsRβ and promotes its degradation, were elevated in skeletal muscle of PPARβ/δ-deficient mice. However, treatment with the PPARβ/δ agonist reduced EphB4 levels in normal mice, further supporting its potential role in improving insulin sensitivity.
“This study identifies novel mechanisms through which PPARβ/δ regulates InsRβ protein levels in skeletal muscle, providing new insights into its beneficial effects on insulin resistance and DM2,” concluded Professor Vázquez-Carrera.
Future Directions
These findings pave the way for further research into PPARβ/δ as a therapeutic target for insulin resistance and DM2. The ability to modulate InsRβ protein levels through pharmacological interventions could lead to innovative treatments that improve glucose metabolism and prevent diabetes-related complications.
For more details, refer to the full study: Jue-Rui Wang et al, PPARβ/δ upregulates the insulin receptor β subunit in skeletal muscle by reducing lysosomal activity and EphB4 levels, Cell Communication and Signaling (2024). DOI: 10.1186/s12964-024-01972-5.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Readers are encouraged to consult healthcare professionals for diagnosis and treatment of medical conditions.