August 25, 2024 – A groundbreaking study published in Nature Communications has identified a new player in Huntington’s disease (HD), a debilitating genetic neurodegenerative disorder. Researchers have implicated the gene CHCHD2 as a novel therapeutic target and uncovered its role in the disease’s progression. The study, involving six labs from the Max Delbrück Center and Heinrich Heine University Düsseldorf (HHU), represents a significant advancement in understanding Huntington’s disease and its underlying mechanisms.
The collaborative research team, led by Dr. Jakob Metzger of the “Quantitative Stem Cell Biology” lab and Professor Alessandro Prigione of the “Stem Cell Metabolism” lab at HHU, explored the effects of Huntington’s disease mutations using brain organoid models. Their findings reveal that mutations in the HTT gene, responsible for Huntington’s disease, also impact CHCHD2, a gene crucial for mitochondrial function.
Dr. Pawel Lisowski, co-lead author from the Max Delbrück Center, expressed surprise at the discovery that Huntington’s disease can disrupt early brain development through mitochondrial dysfunction. “Our study highlights the importance of early detection, as the organoid model shows that HTT mutations may impair brain development even before clinical symptoms appear,” added Selene Lickfett, co-lead author and doctoral student at HHU.
The Role of Brain Organoids
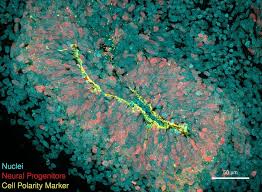
Brain organoids, three-dimensional structures grown from stem cells, offer an unprecedented view into cellular interactions and disease mechanisms. These models, though small, mimic early-stage human brains and have provided valuable insights into Huntington’s disease. Researchers observed that in organoids derived from stem cells with HTT mutations, the CHCHD2 gene was under-expressed, leading to reduced neuronal cell metabolism. While CHCHD2 has been previously linked to Parkinson’s disease, this is the first time it has been associated with Huntington’s disease.
The team successfully reversed the adverse effects on neuronal cells by restoring CHCHD2 function, suggesting that targeting this gene could be a viable therapeutic strategy. Dr. Metzger noted that these findings challenge the prevailing view that Huntington’s disease primarily affects mature neurons, indicating that pathology might begin earlier than previously thought.
Implications for Future Therapies
The study’s results offer promising new avenues for treatment. Prigione highlighted that genome editing techniques, particularly those targeting the CAG repeat region in the HTT gene, demonstrated potential in reversing developmental defects. Additionally, therapies aimed at increasing CHCHD2 expression could offer new strategies for managing Huntington’s disease.
These findings not only open doors for potential therapies for Huntington’s disease but also have broader implications for other neurodegenerative conditions. “Early interventions that address mitochondrial dysfunction could pave the way for innovative treatments for age-related diseases,” Prigione concluded.
This research marks a significant step forward in the quest to understand and eventually treat Huntington’s disease, offering hope for new therapeutic approaches that target the disease at its molecular roots.