January 8, 2025 — University of Freiburg
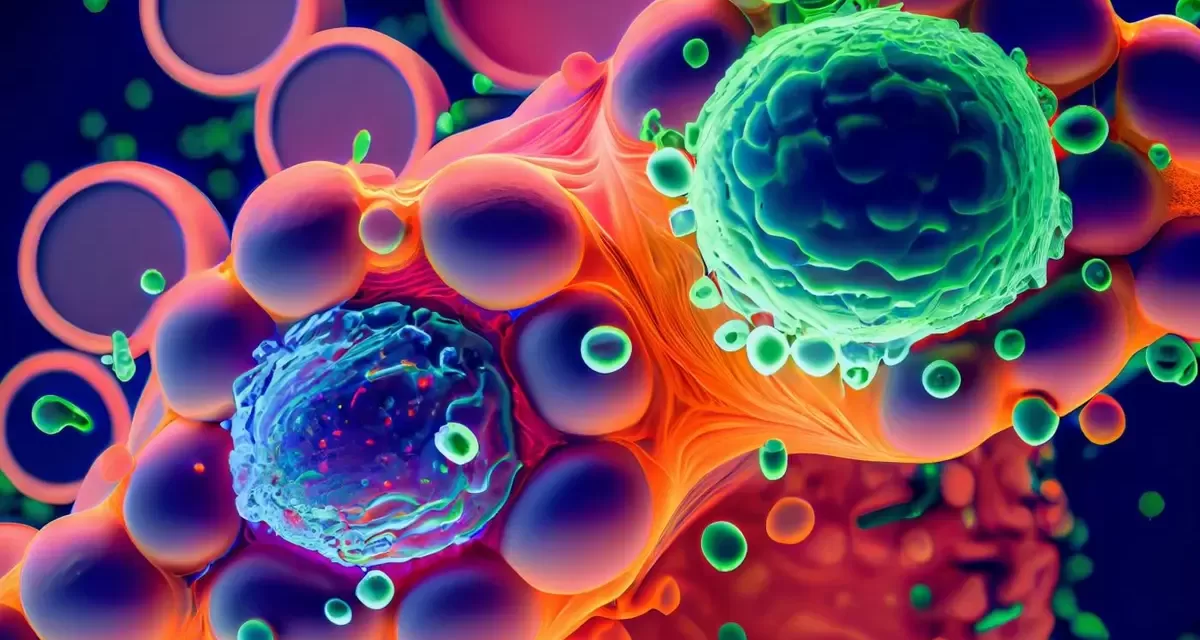
Our cells are constantly recycling and disposing of waste in a process known as autophagy. This essential biological mechanism allows cells to break down unnecessary components and repurpose them, preventing harmful aggregates from forming. It plays a critical role in maintaining cellular health and is linked to numerous diseases, including neurodegenerative disorders like Alzheimer’s and certain cancers.
A groundbreaking study led by Prof. Dr. Claudine Kraft from the CIBSS Cluster of Excellence at the University of Freiburg and Dr. Florian Wilfling from the Max Planck Institute of Biophysics in Frankfurt has unlocked new insights into how autophagy begins, with significant implications for medical research.
The research team discovered the specific conditions required for autophagy to kickstart in cells, and they were able to replicate these conditions in yeast cells to trigger the breakdown of molecules previously considered non-degradable. This discovery paves the way for potential therapeutic interventions targeting neurodegenerative diseases and enhancing the effectiveness of cancer treatments.
The Mechanism Behind Autophagy
Autophagy is a process where cellular waste, such as damaged organelles and proteins, is enclosed by membranes and broken down into basic building blocks for reuse. Before this process begins, the waste material must first be identified by specific receptors and adapter molecules. However, the exact mechanisms behind this recognition and initiation were previously unclear.
The research team has revealed that, surprisingly, the receptors must bind weakly to the waste material for autophagy to be triggered. If the binding is too strong, the process does not commence. This unexpected finding was demonstrated through computer simulations and experiments with living yeast and human cells.
“The weak binding allows the receptors to stay mobile and form clusters,” explained Kraft. “Once a critical concentration is reached, phase separation occurs, and a droplet forms, similar to oil in water. This droplet creates a flexible platform for other molecules involved in the autophagy process.”
Controlling the Process for Medical Benefit
The researchers further tested their findings by introducing virus particles into yeast cells, particles that the cells normally cannot break down. By modifying the surface of the virus particles to allow weak binding to autophagy receptors, they successfully triggered the degradation of the viral proteins. When the virus particles were altered to bind strongly to the receptors, degradation did not occur.
This discovery offers a promising pathway for future medical treatments. By controlling how autophagy is triggered, researchers could potentially target the breakdown of harmful aggregates in diseases like Alzheimer’s or even enhance the response to cancer therapies.
“The ability to specifically intervene in the autophagy process offers a new avenue for therapeutic strategies,” noted Wilfling. “We are hopeful that our findings will lead to more effective treatments for diseases associated with protein aggregation and dysfunction.”
Supporting Research and Funding
The study was supported by the German Research Foundation (DFG), the European Research Council under the Horizon 2020 programme, the Max Planck Society, and the European Union. The results were published in the prestigious scientific journal Nature Cell Biology, and represent a significant advance in our understanding of cellular recycling mechanisms.
As the research progresses, the potential to manipulate autophagy offers hope for new approaches to treating a variety of diseases, making the study a pivotal contribution to the fields of cell biology, biomedicine, and therapeutic development.