Researchers from Cologne, Bochum, Padova, and Angers have made a groundbreaking discovery linking mitochondrial function, protein quality control, and cellular health. Their findings suggest that the failure of these processes may be a key driver behind Charcot-Marie-Tooth disease (CMT), a currently incurable neurological disorder.
Led by Mafalda Escobar from the University of Cologne’s Institute of Genetics, CECAD Cluster of Excellence in Aging Research, and Center for Molecular Medicine Cologne (CMMC), the study has revealed a previously unknown function of the mitochondrial protein Mitofusin 2 (MFN2). The research, published in Nature Communications, could have significant implications for the treatment of CMT and similar conditions.
The Unexpected Role of MFN2
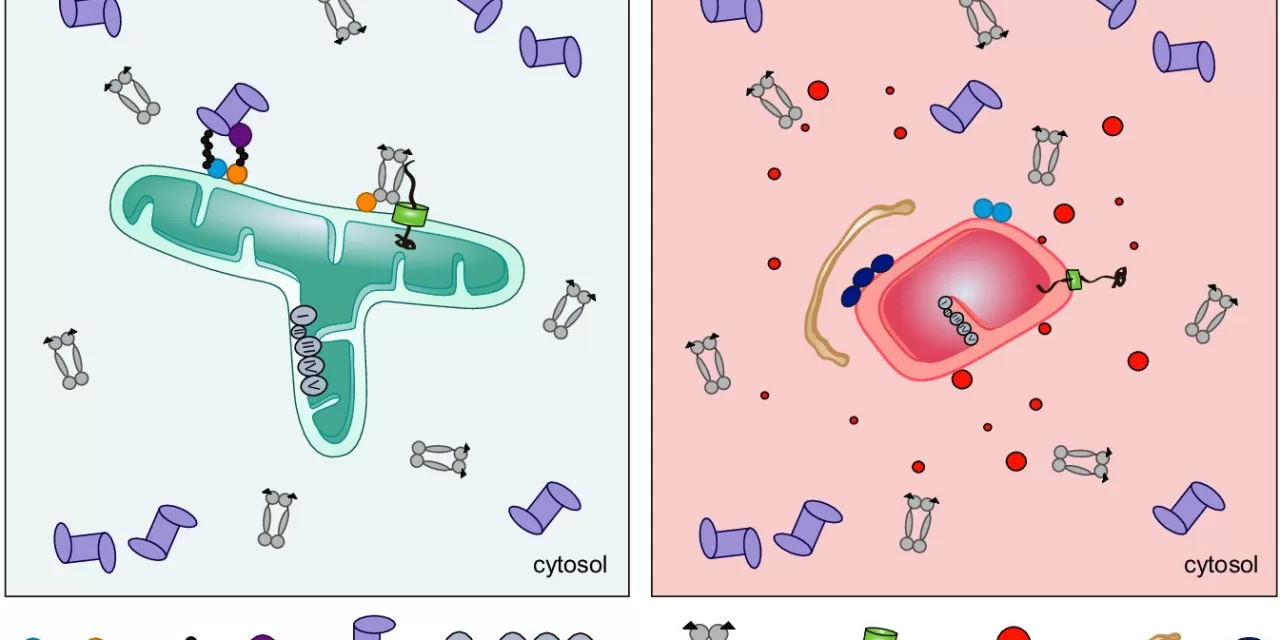
Mitochondria, often referred to as the powerhouse of the cell, play essential roles beyond energy production, including metabolism regulation, gene expression, and cell survival. MFN2 has been well-known for its role in mitochondrial fusion, but this study uncovers a novel function—maintaining protein quality within cells.
The researchers found that MFN2 interacts with the proteasome and molecular chaperones, cellular mechanisms responsible for preventing newly produced proteins from forming toxic aggregates that can lead to neurodegeneration. When MFN2 is mutated, as seen in CMT patients, this protective function is lost, leading to harmful protein clumping.
Potential for New Therapeutic Approaches
“Although MFN2 is a major gene implicated in CMT, most other genes associated with the disease are not mitochondrial, making it even more surprising that MFN2’s link to CMT is independent of its traditional role in mitochondrial dynamics,” said Mariana Joaquim, one of the study’s first authors.
To further understand MFN2’s unique function, the team compared it to its closely related counterpart, MFN1. While numerous MFN2 mutations have been associated with CMT, MFN1 has not been implicated in the disease. By generating human cell lines lacking either MFN1 or MFN2, the team discovered that only MFN2 interacts with the proteasome and prevents harmful protein accumulation, emphasizing its specialized role in cellular health.
The study employed advanced proteomics, microscopy, and biochemistry techniques, with major support from research facilities at CECAD and the CMMC. Additionally, researchers from the Cologne Graduate School of Ageing Research (CGA) played a crucial role in these findings.
Future Implications
Doctoral researcher Maria-Bianca Bulimaga shared her excitement about the findings: “Seeing these aggregates in CMT patients’ cells was a real eye-opener for me. It reinforced how mitochondria are deeply involved in balancing protein synthesis and degradation. It’s something I’m eager to explore further.”
The study suggests that MFN2’s function in protein quality control could also be relevant for conditions such as obesity, where cellular stress and protein misfolding contribute to disease progression. “Understanding how MFN2 interacts with cellular machinery that maintains protein health may help us develop treatments that prevent harmful protein aggregation and protect neuronal function in CMT and other neurodegenerative diseases,” Escobar stated.
The research was supported by the German Research Foundation (DFG), as well as the Fritz Thyssen, Boehringer Ingelheim, and Bayer Foundations.
For more information: Mariana Joaquim et al., “Mitofusin 2 displays fusion-independent roles in proteostasis surveillance,” Nature Communications (2025). DOI: 10.1038/s41467-025-56673-5.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Readers should consult healthcare professionals for diagnosis and treatment of any medical conditions.