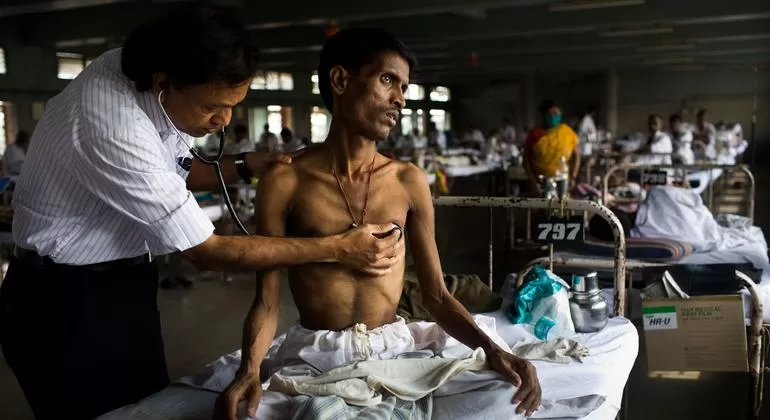
January 8, 2025 – A significant breakthrough in the fight against tuberculosis (TB) has been announced, with newly updated guidelines recommending much shorter and all-oral treatment regimens for both children and adults diagnosed with TB. This change comes after years of research and concerted efforts to improve the effectiveness and accessibility of TB treatment.
The World Health Organization (WHO) had previously reported a worrying surge in global TB cases, with 8.2 million new diagnoses in 2023, marking the highest numbers since global TB monitoring began in 1995. The long and cumbersome treatment regimens have been one of the major barriers to successful TB management worldwide. Traditionally, TB treatment required a lengthy regimen lasting from six months to over a year, which has contributed to poor patient adherence and the development of drug-resistant strains.
However, the American Thoracic Society (ATS), the Centers for Disease Control and Prevention (CDC), the European Respiratory Society (ERS), and the Infectious Diseases Society of America (IDSA) have collaborated to release new clinical practice guidelines. The guidelines, published on December 30, 2024, in the American Journal of Respiratory and Critical Care Medicine, introduce shortened, all-oral regimens that are expected to drastically reduce treatment times.
Dr. Jussi Saukkonen, MD, ATSF, one of the lead authors of the new guidelines, explained, “For decades, we saw little drug development for TB treatment. With recent advancements, we’ve managed to shorten the treatment duration for both drug-susceptible and drug-resistant TB to just four and six months, respectively, for most patients.”
The updated guidelines focus on streamlining treatments for both children and adults. For adolescents and adults with drug-susceptible pulmonary TB, a new four-month regimen has been recommended, replacing the previous six-month protocol. The new treatment involves two months of isoniazid, rifapentine, pyrazinamide, and moxifloxacin, followed by two months of isoniazid, rifapentine, and moxifloxacin.
The guidelines also provide a similar update for children and adolescents with non-severe, drug-susceptible pulmonary TB, advocating for a four-month treatment regimen instead of the traditional six months.
For patients with drug-resistant TB, the recommendations suggest the use of a six-month regimen of bedaquiline, pretomanid, and linezolid (BPaL), replacing the current 15-month or longer treatment plans for rifampin-resistant TB. This shortened regimen is expected to improve treatment adherence and reduce the overall burden on healthcare systems, especially in low-resource settings.
Despite these advances, the expert panel cautioned that careful adherence to treatment is crucial to prevent the emergence of drug resistance. Dr. Saukkonen emphasized the importance of directly observed treatment (DOT), close monitoring, and drug-susceptibility testing to ensure the success of TB treatment programs globally.
The new recommendations are expected to be a game-changer in the global fight against TB, offering faster and more manageable treatment options for millions of people. However, experts stress that the success of these regimens depends heavily on patient adherence and the monitoring of potential side effects.
For more information on the updated TB clinical practice guidelines, visit the ATS website or read the full guidelines in the American Journal of Respiratory and Critical Care Medicine.