Rockefeller University researchers identify rare mutation linked to tuberculosis vulnerability
A groundbreaking discovery by a team of US researchers from The Rockefeller University may redefine how inflammatory disorders are treated, shedding light on a rare mutation that dramatically increases susceptibility to tuberculosis (TB) while leaving carriers unaffected by other infectious diseases. The study, published Wednesday in Nature, challenges long-held views about the immune system and the role of a pro-inflammatory cytokine, TNF (tumor necrosis factor), in the body’s defense mechanisms.
For decades, TNF has been recognized for its critical role in orchestrating the immune response, with a deficiency of this cytokine known to heighten the risk of developing TB. However, this latest research, led by Stephanie Boisson-Dupuis and Jean-Laurent Casanova, uncovered a genetic cause of TNF deficiency and revealed its surprising and highly specific impact on lung immunity.
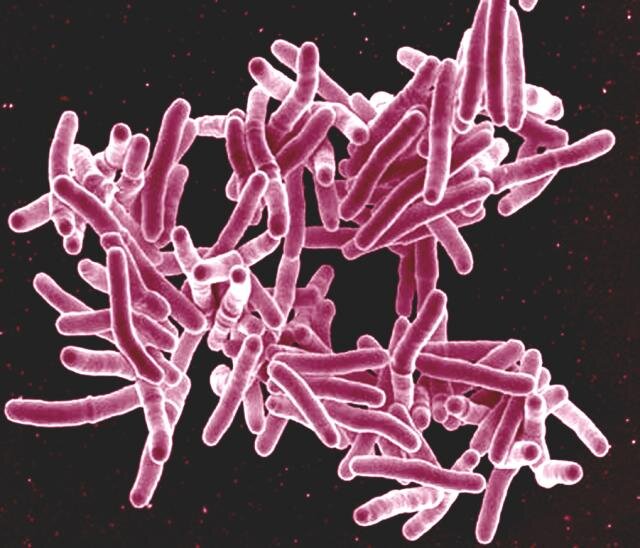
The study focused on two patients from Colombia — a 28-year-old woman and her 32-year-old cousin — who suffered from recurring TB infections despite initial successful treatments. Both patients, repeatedly hospitalized with severe lung conditions, shared a genetic mutation in the TNF gene, which impaired their ability to generate reactive oxygen species (ROS) through a critical immune process called the respiratory burst. This, in turn, allowed Mycobacterium tuberculosis (Mtb), the bacterium responsible for TB, to overrun their lung cells, particularly alveolar macrophages.
“Our findings indicate that TNF’s role may be far more limited than previously believed, primarily focused on protecting the lungs from TB,” said Boisson-Dupuis. This insight has the potential to change the way clinicians view and treat immune-related conditions, especially those involving TNF.
TNF’s Refined Role in Immunity
While TNF has been associated with a variety of inflammatory functions, Casanova suggests that its primary importance may lie in safeguarding against TB, particularly within the lungs. “The past 40 years of scientific literature have attributed a wide variety of pro-inflammatory functions to TNF,” he said. “But beyond protecting the lungs against TB, it may have a limited role in inflammation and immunity.”
This revelation could have far-reaching clinical implications, especially for individuals undergoing treatment with TNF inhibitors — drugs commonly used for autoimmune and inflammatory diseases like rheumatoid arthritis and Crohn’s disease. The study provides a key explanation for the increased TB risk among patients using these medications, which inhibit TNF activity.
Solving an Immunological Mystery
The researchers suspect that this newly identified mutation is just one of several rare genetic variants that leave some people more vulnerable to TB. They previously identified a mutation in the CYBB gene, which disables the respiratory burst across immune cells and hinders the production of ROS. The current discovery, however, ties TNF directly to the regulation of this critical immune defense.
In the two Colombian patients, the defective TNF gene blocked ROS production in their lung cells, preventing them from mounting a robust defense against Mtb. This deficiency allowed the bacterium to persist in their lungs, causing recurrent, severe TB infections despite temporary improvement with antibiotics.
“This discovery not only unravels a mystery about the link between TNF and TB but also points to new possibilities for treating inflammatory diseases,” added Boisson-Dupuis.
Potential for New Therapies
With the study’s revelations, scientists are hopeful that future treatments for both TB and inflammatory disorders can be refined. Understanding how TNF regulates the respiratory burst and how its absence can predispose individuals to TB could lead to targeted therapies that enhance immunity against TB without compromising the broader immune system.
This research opens a new frontier in the treatment of inflammatory conditions, offering hope for individuals with rare genetic vulnerabilities while reshaping the future of TB treatment and immune system modulation.