In a groundbreaking development, scientists at Scripps Research and the Albert Einstein College of Medicine have designed innovative drug-like molecules that can prevent influenza infection at its very first stage. These inhibitors, which target the virus before it can enter the body’s respiratory cells, represent a significant advancement in flu prevention and treatment.
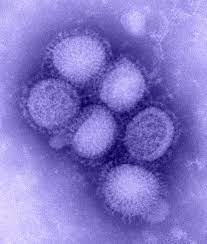
Published in the Proceedings of the National Academy of Sciences on May 16, 2024, the study introduces inhibitors that block hemagglutinin, a protein on the surface of type A influenza viruses. By targeting hemagglutinin, the inhibitors prevent the virus from attaching to and entering respiratory cells.
“We’re trying to target the very first stage of influenza infection since it would be better to prevent infection in the first place, but these molecules could also be used to inhibit the spread of the virus after one’s infected,” says Ian Wilson, DPhil, the Hansen Professor of Structural Biology at Scripps Research and a corresponding author of the study.
While the inhibitors require further optimization and testing before they can be assessed as antivirals in humans, the researchers are optimistic about their potential. Unlike annual flu vaccines, these inhibitors might not need yearly updates, providing a more consistent and long-term solution to flu prevention and treatment.
The research builds on previous work where the team identified a small molecule, F0045(S), with limited ability to bind and inhibit H1N1 influenza viruses. Using a high-throughput hemagglutinin binding assay, they screened large libraries of small molecules and identified F0045(S) as a lead compound.
To enhance its effectiveness, the team utilized “SuFEx click-chemistry,” a method developed by two-time Nobel laureate and co-author K. Barry Sharpless, PhD. This process generated a large library of candidate molecules, leading to the discovery of two promising compounds, 4(R) and 6(R), with superior binding affinity.
Wilson’s lab produced X-ray crystal structures of these compounds bound to the flu hemagglutinin protein, revealing their binding sites and mechanisms. “We showed that these inhibitors bind much more tightly to the viral antigen hemagglutinin than the original lead molecule did,” explains Wilson. “By using click-chemistry, we basically extended the compounds’ ability to interact with influenza by making them target additional pockets on the antigen surface.”
Further testing in cell culture confirmed that 6(R) was non-toxic and had more than 200-times improved antiviral potency compared to F0045(S). The team then optimized 6(R) to develop compound 7, which demonstrated even better antiviral properties.
“This is the most potent small-molecule hemagglutinin inhibitor developed to date,” says Seiya Kitamura, corresponding author and now an assistant professor at the Albert Einstein College of Medicine.
Future studies will focus on optimizing compound 7 and testing it in animal models. “In terms of potency, it will be hard to improve the molecule any further, but there are many other properties to consider and optimize, for example, pharmacokinetics, metabolism, and aqueous solubility,” says Kitamura.
As the inhibitors currently target only H1N1 strains, researchers are also working to develop similar inhibitors for other strains like H3N2 and H5N1. This innovative approach could potentially transform flu treatment and prevention, offering hope for more effective and lasting solutions against seasonal influenza.
This research was supported by the NIH, the Nathan Shock Institute of Aging Research, and Einstein-Montefiore.