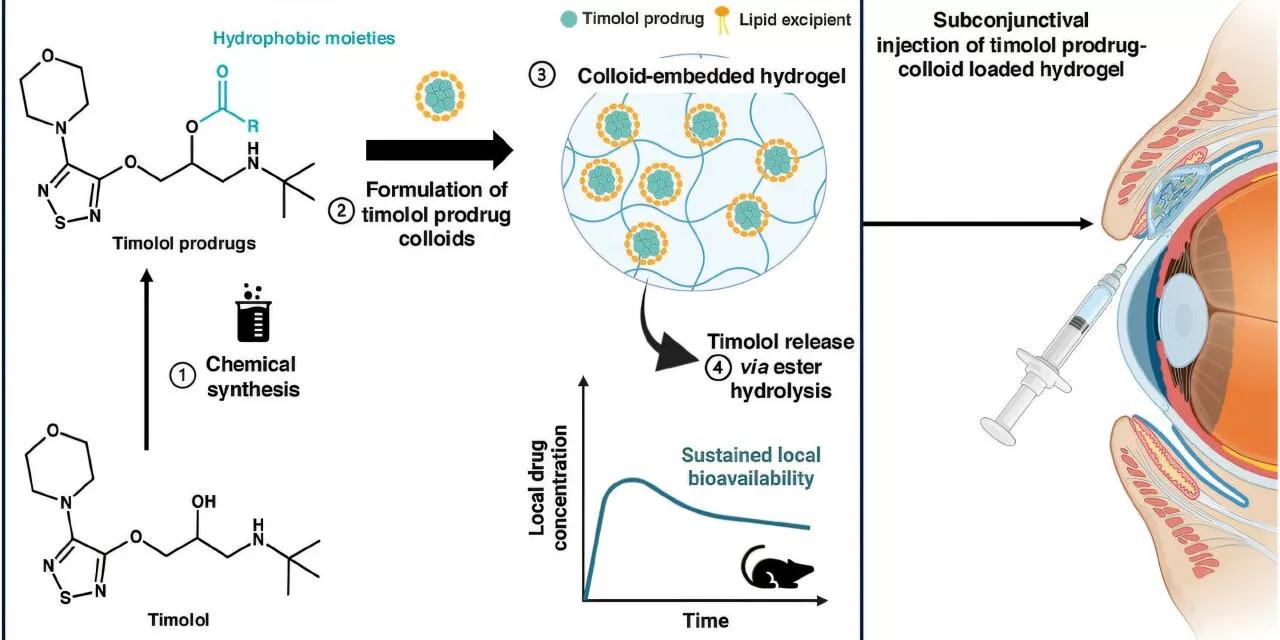
Toronto, Canada – A groundbreaking drug delivery method could revolutionize glaucoma treatment, potentially replacing months of daily eye drops with a single injection under the eyelid. Researchers at the University of Toronto have developed a novel approach using colloidal drug aggregates (CDAs) to extend the effects of a small-molecule glaucoma drug from six hours to up to seven weeks.
The research team, led by Professor Molly Shoichet from the department of chemical engineering and applied chemistry and the Institute of Biomedical Engineering, published their findings in the journal Advanced Materials.
Addressing Challenges in Glaucoma Treatment
Glaucoma, a leading cause of blindness, is a group of eye diseases that increase intraocular pressure, damaging the optic nerve. While current treatments, including eye drops and invasive surgeries, help slow the disease’s progression, patient compliance and efficacy remain significant challenges—especially for older adults who often struggle with self-administering eye drops at precise intervals.
“Eye drops are the most common treatment for glaucoma, but they come with issues regarding efficacy and patient compliance,” explained Mickaël Dang, a postdoctoral fellow in Shoichet’s lab and the study’s first author. “Self-administering drops perfectly can be difficult, and their effects are transient. Laser therapies and surgical treatments require injections inside the eye every few months, posing risks of complications such as infection, inflammation, or vision loss.”
A Novel Approach: Colloidal Drug Aggregates and Hydrogels
The new method involves modifying timolol prodrug colloids and dispersing them in a hydrogel, enabling slow drug release over several weeks. This innovation not only enhances efficacy—resulting in a 200-fold increase—but also maintains the formulation within the subconjunctival space, reducing the risk of leakage and enhancing drug retention.
CDAs, previously viewed as a hindrance in drug research due to their impact on enzyme- and cell-based assays, are now being repurposed for targeted and sustained drug delivery. “We showed that this colloidal drug aggregate could be dispersed in an in situ-forming hydrogel into the subconjunctival space,” said Shoichet. “The hydrogel allows for prolonged drug release while preventing leakage, a common issue with traditional delivery methods.”
Towards Clinical Application
The research team collaborated with Jeremy Sivak, the glaucoma research chair at the Krembil Research Institute, part of the University Health Network, to advance their preclinical studies. With promising results, they are now focused on optimizing the formulation for clinical trials.
“We envision a future where this non-invasive injection can be administered once every month or two in a medical office,” said Dang, who is also affiliated with Synakis, a biotechnology spinoff company founded from Shoichet’s lab research.
“There is a lot of work ahead,” added Shoichet. “We are focusing on product stability and manufacturability while also seeking funding to accelerate the path to clinical use.”
Disclaimer: The information presented in this article is based on preclinical research and has not yet undergone clinical trials. Patients should consult healthcare professionals for current treatment options. The efficacy and safety of this method in humans remain under investigation.