Cleveland, OH – Researchers at Case Western Reserve University have developed a groundbreaking method to detect inflammation using antibodies, paving the way for blood tests that could identify disease-specific biomarkers for conditions such as heart disease, Alzheimer’s disease, and various cancers.
Nearly every disease has an inflammatory component, but current blood tests lack the ability to pinpoint inflammation in specific organs or tissues. The new approach, led by Greg Tochtrop, professor of chemistry at Case Western Reserve, offers a promising solution by detecting inflammation-related compounds that accumulate in affected tissues. The findings were published in the Proceedings of the National Academy of Sciences.
Tracing Inflammation Through Reactive Oxygen Species
Inflammation leaves molecular traces in the body. When immune cells produce reactive oxygen species (ROS) to fight infections, these highly reactive chemicals can also damage DNA, proteins, and lipids. ROS exposure from environmental factors like ultraviolet light, pollution, radiation, and smoking can further contribute to tissue damage.
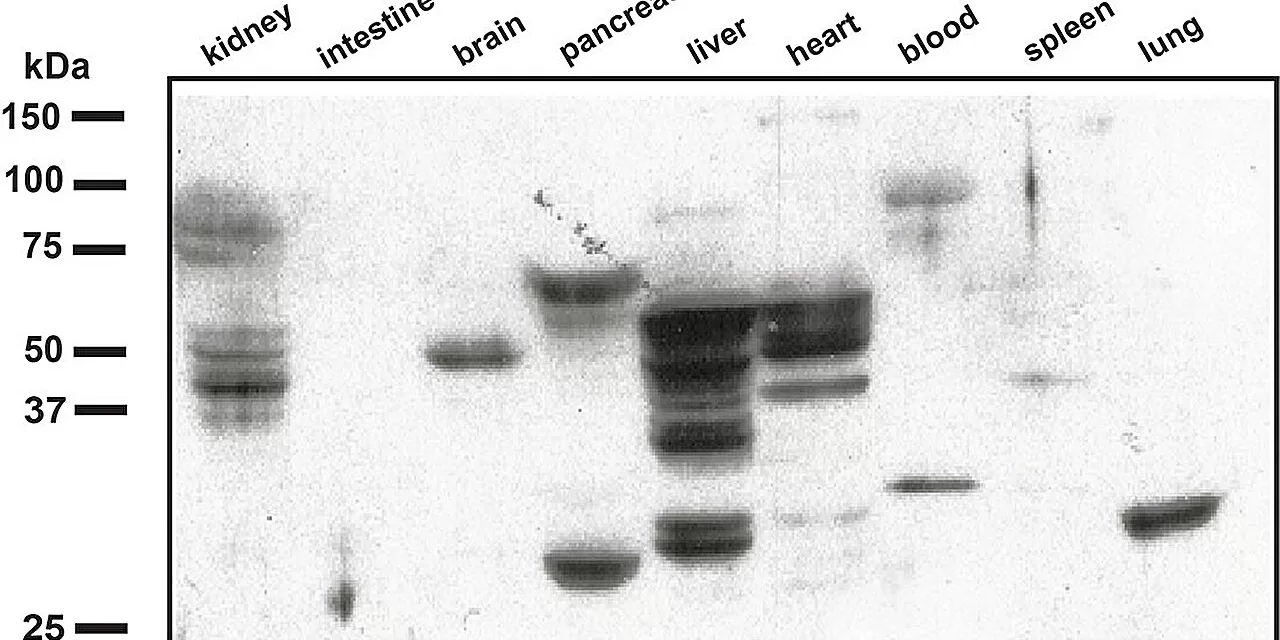
Tochtrop and his team focused on how ROS interacts with linoleic acid, a fatty acid found in all cell membranes, forming compounds called epoxyketooctadecanoic acids (EKODEs). These EKODEs react with cysteine, an amino acid, creating stable bonds that accumulate in tissues experiencing oxidative stress, such as the brain, heart, and liver. Using mouse models, the researchers successfully developed antibodies targeting EKODEs, allowing them to track their buildup in various tissues.
Potential for Disease-Specific Biomarker Detection
The ultimate goal of the research is to correlate different EKODEs with specific diseases, enabling early diagnosis through a simple blood test. According to Tochtrop, this method could function similarly to the A1C test for diabetes, which measures glucose-coated hemoglobin to assess blood sugar levels over time.
One area of interest is identifying EKODE biomarkers in the eye that may indicate conditions like age-related macular degeneration or diabetic retinopathy. Since these biomarkers had not been previously identified, the research team had to develop novel laboratory tools to detect them.
“We had to build the framework for finding these markers from scratch,” Tochtrop said. “This is a prime example of how fundamental research leads to clinically relevant discoveries.”
Implications for Drug Discovery
Beyond diagnostics, this research could aid drug discovery, particularly in targeting reactive cysteines, which play a crucial role in pharmaceutical development. Drug developers could leverage this approach to identify reactive cysteines linked to inflammation, potentially leading to new therapeutic interventions.
“Identifying reactive cysteines is central to drug discovery right now,” Tochtrop explained. “This technique could uncover key targets for drug development, making it a valuable byproduct of our work.”
Looking Ahead
The next phase of the study involves refining antibody specificity to different EKODE compounds and testing their diagnostic potential across various diseases. If successful, this method could transform the way inflammatory conditions are diagnosed and treated, ultimately improving patient outcomes.
Disclaimer
This article is for informational purposes only and does not constitute medical advice. While this research shows promise, further clinical validation is required before the method can be used in routine diagnostics or treatment. Individuals should consult healthcare professionals for medical concerns and rely on approved diagnostic methods for disease detection.