January 10, 2025
In a breakthrough for tuberculosis (TB) treatment, a new antibiotic has shown significant promise in combating the disease, according to a study conducted by the PanACEA consortium, a European-African network of TB researchers. The findings, recently published in The Lancet Microbe, mark a critical step forward in addressing one of the world’s deadliest infectious diseases.
Tuberculosis continues to pose a global health threat, with 10.6 million new infections and 1.3 million deaths reported in 2022 alone. The growing prevalence of antibiotic-resistant TB strains has made the development of new treatments a pressing priority.
“We urgently need an effective new drug to fight tuberculosis, especially in view of the growing problem of antibiotic-resistant strains,” emphasized Dr. Julia Dreisbach, Scientific Program Manager at LMU University Hospital’s Tropical Institute. Together with Professor Michael Hoelscher, Director of the Institute of Infectious Diseases and Tropical Medicine, Dr. Dreisbach is spearheading efforts to develop innovative TB treatments.
At the center of this new hope is BTZ-043, an antibiotic co-developed by the Institute of Infectious Diseases and Tropical Medicine at LMU University Hospital and the Leibniz Institute for Natural Product Research and Infection Biology (Leibniz-HKI) in Jena. The drug targets a critical enzyme required for the cell wall synthesis of Mycobacterium tuberculosis, the bacteria responsible for TB. By disrupting this process, BTZ-043 causes the bacteria to disintegrate and die.
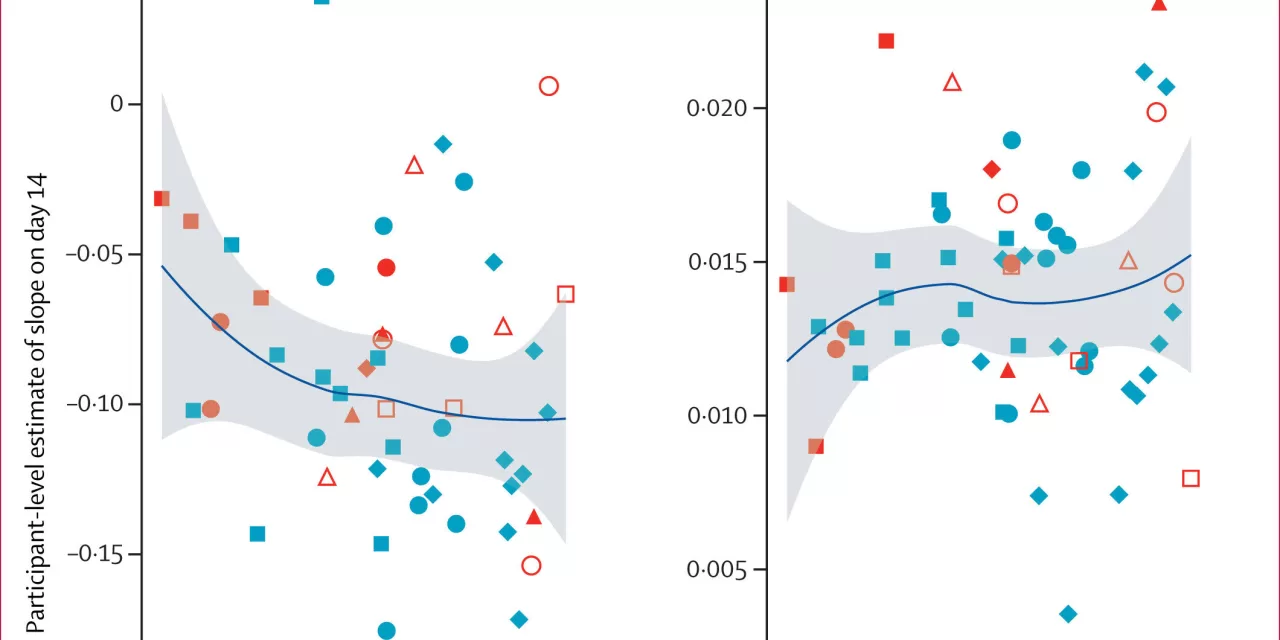
The study, conducted in Cape Town, South Africa, involved 77 adults newly diagnosed with pulmonary TB. The research assessed the safety, tolerability, and antibacterial effectiveness of BTZ-043. “The study demonstrates the antibacterial efficacy and tolerability of BTZ-043 and shows that it can be administered in combination with other tuberculosis drugs,” said PD Dr. Norbert Heinrich, Senior Physician and Scientific Lead for Tuberculosis at LMU.
PanACEA employed an innovative adaptive model-based study design to optimize dosing, marking the first trial of its kind to be carried out in Africa. The design also explored how factors like food intake and drug interactions influence the antibiotic’s performance. “This approach allowed us to obtain a comprehensive understanding of the optimal administration of BTZ-043,” Heinrich explained.
The potential of BTZ-043 as a cornerstone in future TB treatments is generating optimism in the scientific community. If further trials confirm its efficacy and safety, the drug could revolutionize the global fight against TB, offering hope to millions affected by the disease.
For more details, the study is available in The Lancet Microbe:
Norbert Heinrich et al., “Safety, bactericidal activity, and pharmacokinetics of the antituberculosis drug candidate BTZ-043 in South Africa (PanACEA-BTZ-043–02): an open-label, dose-expansion, randomised, controlled, phase 1b/2a trial.” DOI: 10.1016/j.lanmic.2024.07.015