A groundbreaking study has identified nanoplastics, particularly those derived from single-use plastic bottles (SUPBs), as contributors to the alarming spread of antibiotic resistance (AR). The findings underscore a previously unrecognized public health risk, adding a new dimension to the growing global concerns about plastic pollution and AR.
The research, conducted by scientists from the Institute of Nano Science and Technology (INST) Mohali, an autonomous institution under India’s Department of Science and Technology (DST), reveals how plastic nanoparticles can affect bacterial communities, including those in the human gut microbiome. The team, led by Dr. Manish Singh, focused on Lactobacillus acidophilus, a key component of gut health, to investigate whether nanoplastics could transform beneficial bacteria into carriers of AR genes.
Nanoplastics as Agents of Antibiotic Resistance
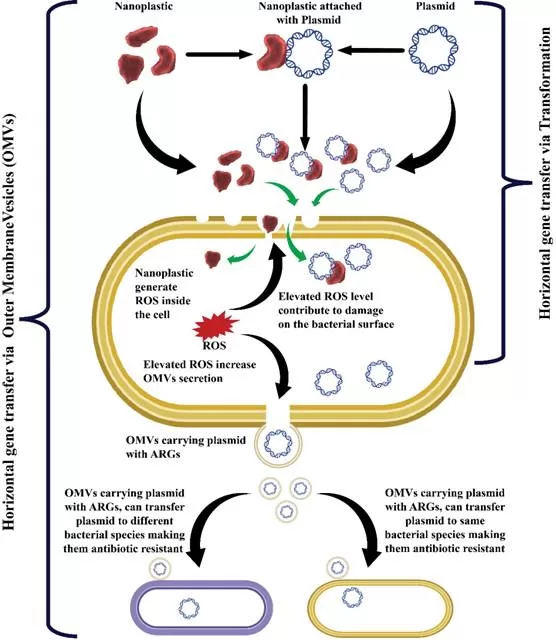
The researchers synthesized environmentally relevant nanoplastics from discarded polyethylene terephthalate (PET) bottles to simulate real-world pollutants. These PET bottle-derived nanoplastics (PBNPs) were found to facilitate the horizontal transfer of AR genes between bacterial species. Specifically, they enabled the transfer of AR genes from E. coli to Lactobacillus acidophilus through a process known as horizontal gene transfer (HGT).
Two novel mechanisms were identified:
- Direct Transformation Pathway: PBNPs act as physical carriers, transporting AR plasmids across bacterial membranes and directly promoting gene transfer.
- OMV-Induced Transfer Pathway: PBNPs induce oxidative stress in bacterial cells, leading to increased secretion of outer membrane vesicles (OMVs). These OMVs, carrying AR genes, serve as potent vectors for gene transfer between unrelated bacterial species.
This discovery highlights the dual role of nanoplastics—not only as pollutants but also as facilitators of microbial gene exchange, posing a significant threat to human health.
Implications for the Human Gut Microbiome
The study, published in the journal Nanoscale, reveals that beneficial bacteria such as Lactobacillus acidophilus could act as reservoirs for AR genes. These genes may subsequently transfer to pathogenic bacteria during infections, amplifying the risks of treatment-resistant diseases.
Protecting beneficial gut bacteria is crucial for maintaining immune function, aiding digestion, and preventing diseases. However, the infiltration of nanoplastics into microbial communities poses a direct challenge to these protective roles, potentially destabilizing the gut microbiome and facilitating the spread of AR.
A Call for Action
The findings underscore the urgent need for stricter safety guidelines, awareness campaigns, and policies addressing plastic pollution and waste management. Mitigating nanoplastic contamination could preserve the integrity of gut microbiota, reduce the risk of AR gene transfer, and support the overall resilience of the human microbiome.
As plastic pollution continues to escalate, this study serves as a stark reminder of its far-reaching consequences. Addressing this emerging threat requires a concerted effort to promote responsible plastic use, enhance waste disposal systems, and prioritize research into mitigating its impacts on human health and the environment.
By tackling these interconnected challenges, society can work towards a future where the dangers of nanoplastics and antibiotic resistance are mitigated, safeguarding both public health and environmental stability.