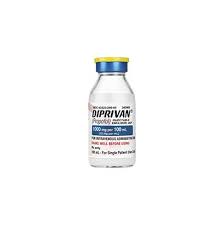
Falsified DIPRIVAN (Propofol) identified in WHO region of the Americas
This WHO Medical Product Alert refers to falsified DIPRIVAN (propofol), identified in Venezuela (Bolivarian Republic of) in July 2022 and reported to WHO in August 2022.
DIPRIVAN is indicated for use as a short-acting intravenous general anaesthetic during diagnostic and surgical procedures, and for the sedation of ventilated patients.
Genuine DIPRIVAN is manufactured by ASTRAZENECA for ASPEN. Both ASPEN and ASTRAZENECA have confirmed that the products referenced in this Alert are falsified on the basis that they deliberately/fraudulently misrepresent their identity and source.
DIPRIVAN is manufactured for the Latin American market and is authorized for local use by several countries including Colombia, Dominican Rep, and Ecuador.
The stated Lot numbers are falsified and have never been issued for DIPRIVAN.
Printing and spelling errors have also been identified and the fill line is potentially inconsistent among the identified vials.
The safety, sterility, and quality of the falsified products referenced in this alert are unknown.
DIPRIVAN is administered intravenously and used to sedate patients. These falsified products may therefore pose a particular high risk to patients.
It is important to detect and remove these falsified products from circulation to prevent harm to patients.
Advice to regulatory authorities and the public
WHO requests increased surveillance and diligence within the supply chains of countries and regions likely to be affected by these falsified products.
All medical products must be approved and obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked. Seek advice from a healthcare professional when in doubt.
If you are in possession of these falsified products, please do not use them.
If you have used these products, or you suffered an adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional and report the incident to the National Regulatory Authority or National Pharmacovigilance Centre.
National regulatory/health authorities are advised to immediately notify WHO if these falsified products are discovered in their country.
If you have any information concerning the manufacture and/or distribution of these products, please inform WHO via [email protected]
Please click here for details and photos of the falsified products referenced in this alert.
WHO Global Surveillance and Monitoring System
for Substandard and Falsified Medical Products
For more information, please visit our website
Email: [email protected]