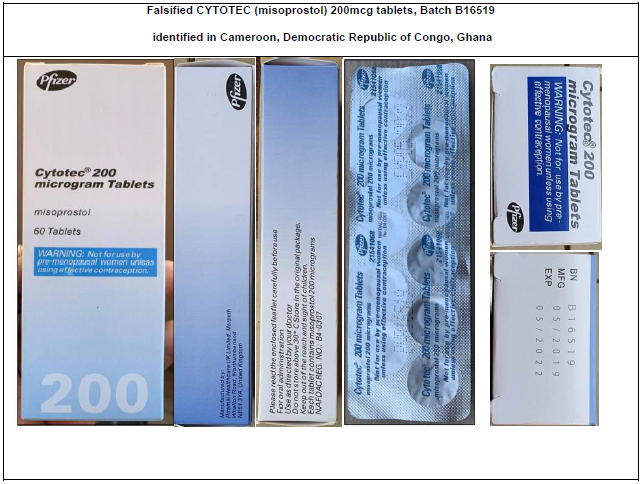
Falsified CYTOTEC identified in WHO region of Africa
Misoprostol is listed on the WHO Model List of Essential Medicines. Genuine Cytotec (misoprostol) is indicated for the treatment of duodenal and gastric ulcers. Other uses of misoprostol as recommended by current WHO guidelines include induction of labour and postpartum haemorrhage prophylaxis, treatment of missed and incomplete miscarriages, induction of abortion, and cervical preparation before uterine instrumentation.
The risk to patient health from falsified Cytotec (misoprostol) is ineffective or delayed treatment for all of the above uses and could also be life-threatening in some circumstances. It is important to detect and remove any falsified Cytotec (misoprostol) from circulation so as to prevent harm to patients.
- Batch B16519 – the batch number does not correspond to genuine manufactured CYTOTEC. Laboratory analysis of samples has also confirmed the product does not contain any active ingredient and does not comply with specifications;
- Batch 14660 – the expiry date (12/2021) on this product is falsified.
Table 1: Products subject of WHO Medical Product Alert N°3/2021

Advice to regulatory authorities and the public
WHO requests increased vigilance within the supply chains of countries and regions likely to be affected by these falsified products. Increased vigilance should include hospitals, clinics, health centers, wholesalers, distributors, pharmacies, and any other suppliers of medical products.
All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
If you are in possession of the above products, please do not use them.
If you have used these products, or you suffered an adverse reaction/event have used these products, you are advised to seek immediate medical advice from a qualified healthcare professional and to report the incident to the National Regulatory Authorities/National Pharmacovigilance Centre.
Table 2: Photographs of products subject of WHO Medical Product Alert N°3/2021