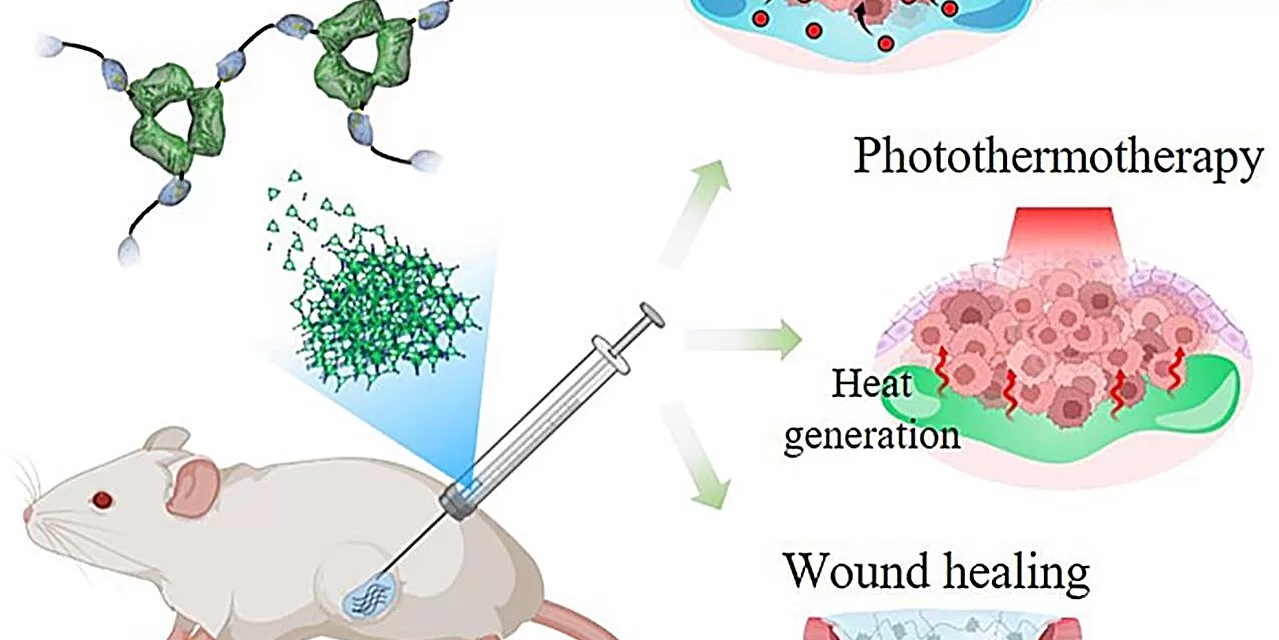
A research team affiliated with UNIST has unveiled a groundbreaking self-standing protein hydrogel that selectively delivers drugs, enhancing medicinal effects while minimizing associated toxicity. This breakthrough has significant implications for cancer treatment and wound healing, offering a controlled and sustained drug release without the need for harmful additives.
Professor Sebyung Kang and his team from the Department of Biological Sciences at UNIST successfully created a self-crosslinkable protein hydrogel. This hydrogel has been designed to leverage natural body proteins to form cross-links, eliminating the need for chemical cross-linkers that often induce cytotoxicity. Their findings, published in the Journal of Controlled Release, highlight the hydrogel’s potential as a safe and effective drug delivery system.
The research was conducted in collaboration with Professor Jinmyoung Joo from the Department of Biomedical Engineering and Professor Chaenyung Cha from the Department of Materials Science and Engineering at UNIST. Their work showcases the hydrogel’s unique ability to gradually release encapsulated drugs as its protein crosslinks decompose.
To enhance the hydrogel’s performance, proliferating cell nuclear antigen proteins were integrated into the formulation to suppress immune inflammatory responses. When tested in mice, the hydrogel showed no signs of immune inflammatory reactions, demonstrating its high biocompatibility.
Furthermore, experiments confirmed the hydrogel’s effectiveness in targeting breast cancer tumors by incorporating anticancer drugs such as doxorubicin, as well as promoting wound healing with growth factors like PDGF-BB. The hydrogel also proved valuable in photothermal chemotherapy, which uses photosensitized particles to generate localized heat when exposed to light, effectively destroying cancer cells.
Professor Kang stated, “The self-crosslinkable protein hydrogel we developed serves as an effective platform for delivering or encapsulating various treatment modalities tailored to specific injection sites.”
Lead researcher Soomin Eom added, “Our study highlights the potential for developing versatile protein hydrogels suitable for a range of biomedical applications.”
Reference:
More information: Soomin Eom et al, In situ forming and self-crosslinkable protein hydrogels for localized cancer therapy and topical wound healing, Journal of Controlled Release (2024). DOI: 10.1016/j.jconrel.2024.12.026
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Further research and clinical trials are required before the hydrogel can be used in medical applications.