- Steps taken by the Government of India for COVID-19 containment and management
The Government of India continued to closely monitor the evolving nature of COVID-19 pandemic in India as well as globally. A close watch was also kept on improving knowledge about the virus, the disease, its long-term impacts, advancements being made in India as well as globally in terms of public health tools, diagnostics, therapeutics and vaccines. The various technical bodies under various Ministries/Departments continued to maintain a close watch over the evolving nature of the causative virus and their public health implications. India continued its graded yet pre-emptive and proactive approach towards COVID-19 management.
The COVID-19 trajectory in India experiences a sharp increase during March-May 2021, however, since May 2021, the trajectory has witnessed a considerable and sustained decline. Owing to Government of India’s five-fold strategy of test-track-treat-vaccinate and COVID appropriate behavior through a Whole of Government & Whole of Society approach, India has been able to limit its cases and deaths per million to 32,775 cases per million and 389 deaths per million population (as on 25th November 2022) respectively, which is one of the lowest in the world as compared to similarly affected countries.
The Hon’ble Prime Minister provided the much required strong and decisive leadership and guidance for national response to the pandemic. The Prime Minister Office and Ministry of Health & Family Welfare has been in regular interactions with the all States and UT administrations to review the preparedness and response measures being taken and also to identify areas for further improvement and coordination. The Committee of Secretaries under Cabinet Secretary took regular reviews with all related Ministries of Health, Defence, Ministry of External Affairs, Civil Aviation, Home, Textiles, Pharma, Commerce and other officials including with State Chief Secretaries.
The Joint Monitoring Group (JMG) under the Chairmanship of DGHS and National Task Force on COVID-19 under ICMR continue to assess the risk, review the preparedness & response mechanisms and finalize technical guidelines.
The Government of India, based on its past experience of successfully managing pandemics and epidemics in the past and the evolving evidence based contemporary knowledge about the disease, provided the requisite strategy, plans and procedures to the State Governments and UT administrations. This includes containment plans and guidelines on a wide range of subjects related to travel, behavioral & psycho-social health, surveillance, laboratory support, hospital infrastructure, clinical management, rational use of Personal Protective Equipment (PPE) etc.
Taking note of the evolving COVID-19 situation globally and emergence of mutant variants of SARS-CoV-2 virus, the guidelines for international arrivals were reviewed from time to time. The last updated guidelines were issued on 21st November 2022.
As per the updated guidelines, all international travellers to India should preferably be fully vaccinated as per the approved primary schedule of vaccination against COVID-19 in their Country. The updated guidelines also prescribe precautionary measures to be followed like preferable use of masks and adherence to physical distancing measures. There is no need to submit any details about COVID-19 vaccination status or RT-PCR testing for traveling to India.
Union Ministry of Health & FW is coordinating and collaborating with other stakeholder Ministries/departments including Ministry of Civil Aviation, Ministry of Ports, Shipping and Waterways, Ministry of Railways etc. Further Port/Airport Health Officers at International ports/ airports have been instructed to ensure strict health screening of all passengers and if passengers found to be symptomatic during screening shall be immediately isolated, taken to a designated medical facility as per health protocol.
Further, the Union Ministry of Health & Family Welfare is in regular interaction with all States/UTs through formal communication as well as through video conferencing. States/UTs have been urged to undertake the following activities:
- Strict monitoring of International travelers in the community.
- Contact tracing of positive individuals & follow up for 14 days.
- Genome sequencing of positive samples through INSACOG Labs in a prompt manner.
- Continued monitoring of areas where clusters of positive cases emerge.
- Further strengthening of COVID-19 testing infrastructure and ensuring early identification of cases through adequate testing across the States.
- Ensure preparedness of health infrastructure (availability of ICU, Oxygen supported beds, ventilators, etc.) and upgrade health infrastructure under ECRP-II including in rural areas and for pediatric cases.
- Commissioning all PSA plants, ensuring sufficient logistics, drugs etc.
- Ensure rapid COVID-19 vaccine coverage.
- Ensuring adherence to COVID Appropriate Behaviour.
The laboratory network is continuously being strengthened progressively in the last two years both in terms of testing infrastructure as well as diagnostics. As of 4th Novemebr 2022, a total of 1453 government laboratories and 1935 Private Laboratories are conducting COVID-19 Testing. At present India is testing around 2 – 2.5 lakh samples a day.
A three-tier arrangement of health facilities was created for appropriate management of COVID-19 cases, [(i) COVID Care Center with isolation beds for mild or pre-symptomatic cases; (ii) Dedicated COVID Health Centre (DCHC) with oxygen supported isolation beds for moderate cases and (iii) Dedicated COVID Hospital (DCH) with ICU beds for severe cases] has been implemented. Tertiary care hospitals under ESIC, Defence, Railways, paramilitary forces, Steel Ministry etc. have been leveraged for case management.
As on 25th November 2022, there are a total of 23988 COVID treatment facilities with 17,93,310 dedicated isolation beds (including 5,15,001oxygen supported isolation beds) and 1,45,014 ICU beds (including 63,850 ventilator beds).
Guidelines on Clinical management of COVID-19 continue to be updated with emerging scientific evidence. The treatment protocol for adults was last updated on 17th January 2022 and has been widely circulated. The mainstay of treatment is supplemental oxygen and other supporting therapy. No specific antivirals have been proven effective. However as per National treatment guidelines, drugs like inhalational Budesonide, Dexamethasone, Methylprednisolone and Low Molecular Weight Heparin have been recommended. In addition, provisions for Investigational Therapies have also been made using Remdesivir, and Tocilizumab for defined sub-group of patients under medical supervision.
Guidelines for management of COVID-19 in children and adolescents were also updated on 20th January 2022. The guideline covers guidance on management of acute presentation of COVID-19 as well as Multisystem Inflammatory Syndrome (MIS-C) in children and adolescents found temporally related to COVID-19.
AIIMS, Delhi and similarly placed institutions of the States are designated Centers of Excellence for wider dissemination of latest advancements in COVID management. Telemedicine services using ‘e-sanjeevani’ for tele-consultation is one among the best practices during COVID times.
States are being supported in terms of supply of logistics including PPE kits, N-95 masks, drugs, ventilators, oxygen cylinders, oxygen concentrators etc. States are also being supported in terms of installation of Oxygen concentrator plants/ PSA (Pressure Swing Adsorption plants) plants.
In order to extend on-ground support to the State and District Health Authorities, Central multi-disciplinary teams are also being deployed to States from where upsurge of cases has been reported.
In terms of financial support to States, During the FY 2020-21, funds to the tune of Rs.8257.88 crore have been released to States/UTs towards the India COVID-19 Emergency Response and Health System Preparedness Package.
In addition, ‘India COVID-19 Emergency Response & Health System Preparedness Package: Phase-II’ has also been approved by the Cabinet with Rs 23,123 crores (with Rs. 15,000 Cr as Central Component & Rs 8,123 Cr as State component) and is being implemented from 1st July 2021. This includes support to State/UT level for ramping up Health Infrastructure including those in rural, tribal and peri-urban areas closer to the community, providing support for procurement of drugs and diagnostics to enhance service delivery at district and sub district levels for management of COVID-19 cases (including paediatric care) and for maintaining a buffer of drugs, support for IT Interventions such as implementation of Hospital Management Information System and expanding access to tele-consultations in all districts, and support for capacity building and training for all aspects of management of COVID-19.
Government of India through National Disaster Management Authority (NDMA) has issued ‘Guidelines to provide for ex-gratia assistance to kin of the deceased by COVID-19’. NDMA has recommended an amount of Rs. 50,000/- per deceased person including those involved in relief operations or associated in preparedness activities, subject to cause of death being certified as COVID-19. The ex-gratia assistance shall be provided by States from State Disaster Response Funds.
With the intent to develop long term capacities in preparedness for future surges of COVID-19 and other public health emergencies, PM Ayushman Bharat Health Infrastructure Mission (PM-ABHIM) has been approved with an outlay of Rs. 64,180 crores over 6 years. The PM-ABHIM envisages increased investments in public health and other health reforms to safeguard against future resurgences of COVID-19, if any, and future public health emergencies by:
- Strengthening of Health and Wellness Centers in villages and cities for early detection of diseases
- Addition of new critical care-related beds at district level hospitals.
- Operationalization of Regional National Centers for Disease Control (NCDC).
- Establishment of metropolitan units in urban areas and BSL-III labs across the country to strengthen the laboratory network.
- Strengthening of existing Viral Diagnostic and Research Labs (VRDLs) and creation of new National institutes of Virology (NIVs) and a National Institute for One Health through ICMR.
- Strengthening of Public Health Units at international Points of Entry (PoEs)
The Government of India will continue to maintain a close watch over the evolving pandemic.
- Ayushman Bharat:
Ayushman Bharat comprises of two components:
- The first component pertains to creation of 1,50,000 Health and Wellness Centres (AB-HWCs) by upgrading the Sub Health Centres (SHCs) and rural and urban Primary Health Centres (PHCs), in both urban and rural areas, to bring health care closer to the community. These centres aim to provide Comprehensive Primary Health Care (CPHC), by expanding and strengthening the existing Reproductive & Child Health (RCH) and Communicable Diseases services and by including services related to Non-Communicable Diseases (common NCDs such as, Hypertension, Diabetes and three common cancers of Oral, Breast and Cervix) and incrementally adding primary healthcare services for mental health, ENT, Ophthalmology, Oral health, Geriatric and Palliative care and Trauma care as well as health promotion and wellness activities like yoga. A few States/UTs have already started rolling out these additional packages in a phased manner.
- The second component is the Ayushman Bharat-Pradhan Mantri Jan ArogyaYojana (AB-PMJAY). Under Ayushman Bharat – Pradhan Mantri Jan Arogya Yojana (AB-PMJAY), around 10.74 crore poor and vulnerable families identified as per Socio-Economic Caste Census are entitled for health cover of Rs. 5.00 lakh per family per year for secondary and tertiary care hospitalization. As on 25th Nov 2022, 3.8 crore hospital admissions have been authorised worth more than ₹47,000 crore, 28,636 hospitals empanelled, 20.02 crore Ayushman cards issued, 33 States/UTs implementing the scheme, approximately 50% of Ayushman card recipients are women and 46% of the hospitals empanelled are private.
- Comprehensive Primary Health Care (CPHC) through Ayushman Bharat Health and Wellness Centres (AB-HWCs) – Ayushman Bharat aims to holistically address health (covering preventive, promotive, curative, rehabilitative and palliative care), at primary, secondary and tertiary level by adopting a continuum of care approach. In the lifetime of an individual, the primary healthcare services cater to 80-90% of the healthcare needs for improved healthcare outcomes and quality of life of the population.
- The Primary Health Care team ensures that community outreach and population enumeration are done for individuals in their catchment area and screened for communicable diseases and non-communicable diseases for early detection and timely referral for accurate diagnosis. The team further ensures that treatment adherence and follow-up care are provided to the patients in the community. These centres are aimed at delivering primary healthcare services closer to the people and be the first point of contact for healthcare provisioning and referral for secondary and tertiary care. Thus, the essential health services along with the provisioning of essential medicines and diagnostics are provided closer to the community through these centres, as a step towards building stronger and resilient primary healthcare systems which cater to the healthcare needs of the population.
2.1 Achievement and Service Delivery at AB-HWCs:
- As reported by the States/UTs on the AB-HWC Portal, 1,41,830 Health & Wellness Centres have been operationalized till 26th December, 2022.
- As per the data update done by the States/UTs in HWC Portal, till date, 29,94,26,521screenings have been done for hypertension and 25,55,27,170 screenings done for diabetes at these AB-HWCs. Similarly, these functional AB-HWCs have done 17,43,31,240 screenings for oral cancer, 5,66,37,370 screenings for cervical cancer in women and more than 8,27,00,336 screenings for breast cancer in women.
- Further, as on 26-12-2022, a total of 1,59,56,351 Yoga/wellness Sessions have been conducted in operational AB-HWCs.
- With the objective to provide quality health services to a patient residing in rural areas,1,09,748 Ayushman Bharat Health and Wellness Centres (AB-HWCs) have tele- consultation model, achieving a total of 7,11,58,968 teleconsultations.
2.2 Human Resources:
NHM has attempted to fill the gaps in human resources by providing nearly 3.32 lakh additional health human resources to the States including 94308 CHOs, 14,880 GDMOs, 4,456 Specialists, 75,041 Staff Nurses, 77,407 ANMs, 52,883 Paramedics, 429 Public Health Managers and 12,948 Programme Management staffs etc. on contractual basis, as on 30th June, 2022. Apart from providing support for health human resource, NHM has also focused on multi skilling of human resources.
NHM also focused on multiskilling of doctors at strategically located facilities identified by the States e.g. MBBS doctors are trained in Emergency Obstetric Care (EmOC), Life Saving Anaesthesia Skills (LSAS) and Laparoscopic Surgery. Similarly, due importance is given to capacity building of nursing staff and auxiliary workers such as ANMs. NRHM also supports co-location of AYUSH services in health facilities such as PHCs, CHCs and DHs. As on 30th June, 2022, a total of 27,606 AYUSH doctors and 4,337 AYUSH paramedics have been deployed in the States with NRHM funding support.
2.3 Mainstreaming of AYUSH:
Mainstreaming of AYUSH has been taken up by allocating AYUSH services in 6,571 PHCs, 2,809 CHCs, 456 DHs, 4,236 health facilities above SC but below block level and 237 health facilities other than CHC at or above block level but below district level, as on 30th June, 2022.
2.4 National Ambulance Services (NAS):
As on date, 34 States/UTs have the facility where people can dial 108 or 102 telephone number for calling an ambulance. Dial 108 is predominantly an emergency response system, primarily designed to attend to patients of critical care, trauma and accident victims etc. Dial 102 services essentially consist of basic patient transport aimed to cater the needs of pregnant women and children though other categories are also taking benefit and are not excluded. Janani Shishu Suraksha Karyakram (JSSK) entitlements e.g., free transport from home to facility, inter facility transfer in case of referral and drop back for mother and children are the key focus of 102 service.
This service can be accessed through a toll-free call to a dedicated call center. As on 30th June, 2022, 1,856 ALS, 16,859 BLS, 3,253 PTV, 17 Boat and 131 Bike, Emergency Response Service Vehicles are supported under NHM, besides 4,867empanelled vehicles for transportation of patients, particularly pregnant women and sick infants from home to public health facilities and back.
2.5 National Mobile Medical Units (NMMUs):
Support to Mobile Medical Units (MMUs) under NHM, now encompassing both NRHM and NUHM, is a key strategy to facilitate access to public health care particularly to people living in remote, difficult, under-served and unreached areas. As on 30th June, 2022, States/UTs have 1,567 mobile medical units which includes mobile medical units, mobile health units, mobile medical/health vans, boat clinics, eye vans/ mobile ophthalmic units, dental vans under NRHM and NUHM.
2.6 Community Participation:
- a) Accredited Social Health Workers:
There are 10.52 lakhs ASHAs selected across the country in rural and urban areas under the NHM who act as a link between the community and the public health system, as on 30th June, 2022. ASHAs are envisaged to be community health volunteer and are entitled to task/activity based incentives. The Union Cabiet has approved increase in amount , for routine and recurring activities under NHM for ASHAs that will now enable ASHAs to receive atleast fixed monthly incentive of Rs. 2000 per month in the country, against Rs 1000 earlier. 9.74 lakh ASHAs have been provided with drug kits and HBNCs kits across the country in rural and urban areas under the NHM.
ASHA Certification: As per NIOS, as on 3rd April 2022, 60,763 ASHA and ASHA Facilitators have been certified.
In the year 2018, the ASHA benefit package was introduced acknowledging significant contribution and commitment of ASHAs. The package providing coverage for:
- Pradhan Mantri Jeevan Jyoti Beema Yojana (PMJJBY) with a benefit Rs. 2.00 Lakh in case of death of the insured (annual premium contributed by GOI).
- Pradhan Mantri Suraksha Beema Yojana (PMSBY) with a benefit of Rs.2.00 lakh for accidental death or permanent disability; Rs. 1.00 lakh for partial disability (annual premium contributed by GOI).
In Addition, Pradhan Mantri Shram Yogi Maan Dhan (PM-SYM) with pension benefit of Rs. 3000 pm after age of 60 years (50% contribution of premium by GOI and 50% by beneficiaries) is also available for ASHA workers.
- Following additional incentives for ASHAs have been approved in the Mission Steering Group of NHM in its 7th Meeting held on 7th September, 2022:
- Provision of a cash award of Rs. 5000/- for each certification to acknowledge The achievement of the ASHAs and ASHA Facilitators who have successfully been certified in two independent certificates- (i) RMNCHA+N (ii) Expanded package of new services from Non-Communicable Diseases to Palliative Care
- Provision of an incentive of Rs. 10/- for ASHAs for each ABHA account created and seeded in various IT portals of MoHFW such as CPHC NCD Portal and RCH Portal etc.
- Provision of incentive at rate of Rs. 50/- to ASHA or community volunteer for facilitating seeding of bank account information of notified TB patient in Ni-kshay portal within 15 days of treatment initiation for enabling DBT payments under the National Tuberculosis Elimination Programme.
- Provision of financial incentive to ASHA/ Community Health Volunteer of Rs. 250/- per individual for successful completion of TB Preventive Treatment.
- Enhancing incentives of ASHA for referring SAM children for admission to NRCs and follow up of NRC discharged children are as under :
-
- For referring SAM child with medical complication to NRCs, ASHA incentive enhanced from Rs. 50/- per child to Rs. 100/- per child.
- For follow up visits of SAM children discharged from NRC, ASHA incentive enhanced from Rs. 100/- per child to Rs. 150/- per child. (Rs 50 per visit for 1st and 4th visit and Rs 25 per visit for 2nd and 3rd visit).
- Additional incentive of Rs. 50/- per SAM child for ASHA n case child is declared free of SAM status after completion of all follow ups.
- Incentivizing ASHA worker for PKOL case detection and complete treatment @ Rs. 500/. per case (Rs. 200/- at the time of diagnosis and Its. 300/- after treatment completion) in all 4 Kala-azar endemic states.
- Enhancing ASHA incentive from Rs 75/- to Rs. 200/- per confirmed case of Malaria for ensuring complete treatment.
-
Way Forward:
-
- Revision of Community Processes Guideline
- Support states in rolling out ASHA certification as per revised strategy
- Orientation of State Team on revised role of CP support structures in context of CPHC Support State’s to use community participation platforms for action on social and environmental determinants of health and to build accountability, especially at HWC level.
- Rogi Kalyan Samiti (Patient Welfare Committee) / Hospital Management Society is a simple yet effective management structure. This committee is a registered society that acts as a group of trustees for the hospitals to manage the affairs of the hospital. Financial assistance is provided to these Committee through untied fund to undertake activities for patient welfare. As on 30th June, 2022, 34,210 Rogi Kalyan Samitis (RKS) have been set up involving the community members in almost all District Hospitals, Sub- divisional Hospitals, Community Health Centres and PHCs.
- VHSNCs: At the Village Level, the Village Health, Sanitation and Nutrition Committee (VHSNC) monitors the services provided by the Anganwadi Worker, the ASHA and the sub-centre. These Committees are envisaged to function under the ambit of the Panchayati Raj Institution with adequate representation from women and weaker sections of the society. The VHSNC acts as a subcommittee or statutory body of the Gram Panchayat. The same institutional mechanism is also mandated in urban areas. VHSNCs are provided an Untied fund of Rs 10,000 on annual basis which are topped up based on expenditure of previous year. More than 5.55 lakh VHSNC have been set up across the country till 30th June, 2022.
2.7 24 X 7 Services and First Referral facilities:
To ensure service provision for maternal and child health, 24×7 services at the PHCs have been made available.
As on 30th June 2022, 11,119 PHCs have been made 24×7 PHCs and 3,117 facilities (including 706DH, 842 SDH and 1569 CHCs & other level) have been operationalized as First Referral Units (FRUs).
Besides, NHM envisages provision of assured and high-quality maternal and child health services to be delivered with dignity and care at public health institutions. GoI launched MCH wings to facilitate assured admission for institutional delivery of all pregnant women. These wings are equipped with obstetric HDUs, ICUs, maternity OT, Labor rooms ensuring respectful maternity care etc. for managing high-risk pregnancies and those requiring C-sections. These centers also have skill labs for training of nurses and doctors for providing high quality and skilled maternity care.
2.8 Mera Aspataal:
Recognizing the need to capture the voice of patients for enhanced patient experience and continued learning, India launched its own centralized IT platform i.e. ‘Mera-Aspataal’/ ‘My Hospital’. ‘MeraAspataal’ is a patient feedback system which was launched in September 2016 with a mandate to integrate Central Government Hospitals (CGHs) & District Hospitals (DHs). It has now been extended upto CHC, Rural & Urban Primary Health Centre and private medical colleges and is currently functional in 34 States/UTs. As of now, on 15th December’22, 10,287 government health facilities and 738 non-governmental health facilities are integrated with Mera-Aspataal in 34 States and UTs.
2.9 Kayakalp:
Kayakalp programme was launched on 15 May 2015 under the ‘Swachh Bharat Abhiyaan’. Kayakalp has received overwhelming response across the nation. Within seven years of its implementation Kayakalp has been able to facelift the public health facilities’ appearance. Kayakalp has made strong influence on the existing hygiene and sanitation conditions of public health facilities. Kayakalp has now been extended to the Health & Wellness Centres in all States/UTs. Total number of Kayakalp Awardee facilities have increased from 100 facilities in the FY 2015-16 to 13,825 facilities in FY 2021-22 (as on 2nd November, 2022)
‘Eco-friendly Awards’ have been introduced under the Kayakalp scheme with an award money of Rs 10 Lakh for DH and Rs 5 Lakh for SDH/CHC. In FY 2021-22, 48 facilities awarded as Eco-Friendly Health facility, as on 2nd November, 2022. Further, 408 DHs, 2152 SDHs/CHCs, 5300 PHCs, 1187 UPHCs, 22 UCHCs, 4756 HWCs have been given Kayakalp Awards in 31 States/UTs.
2.10 Swachh Swasth Sarvatra:
- SwachhSwasthSarvatra is a joint initiative of the Ministry of Health & Family Welfare and Ministry of Drinking Water and Sanitation (Now Ministry of Jal Shakti) to achieve better health outcomes through improved sanitation and increase awareness on healthy lifestyles.
- This initiative was launched in December 2016, to build on and leverage the achievements of the two programmes – Swachh Bharat Mission (SBM) and Kayakalp – of the Ministry of Drinking Water and Sanitation and Ministry of Health and Family Welfare, respectively.
- Based on its result and success in rural areas, ‘’Swachh Swasth Sarvatra’’ was implemented in urban areas in Year 2019. In urban areas it is implemented through joint initiatives of the Ministry of Housing and Urban Affairs and Ministry of Health and Family welfare.
Objectives of the program: –
- Enabling Gram Panchayat, cities and wards, where Kayakalp awardee PHCs/UPHCs are located, in sustaining ODF and promoting healthy behaviour.
- Strengthening CHC/UCHCs/UPHCs in ODF blocks/Wards/Cities to achieve a high level of cleanliness to meet Kayakalp standards through a support of Rs 10.0 L for CHCs/UCHCs and Rs 50K for UPHCs under NHM.
- Build capacity through training in Water, Sanitation and Hygiene (WASH) to nominees from such CHCs and PHCs.
Progress under Swachh Swasth Sarvatra:-
- Under this initiative, one-time grant of Rs. 10 Lakhs is provided to the non-Kayakalp awardee CHC located in the Open Defecation Free (ODF) Blocks as a resource for improving the deficiencies found in Kayakalp assessment, so that by the time the next assessment is due they can become Kayakalp awardee.
- Financial support is provided to 374 health facilities in FY 2022-24 under SSS initiative through annual PIP.
2.11 National Quality Assurance Programme:
Quality in delivery health care services is important for improving the health status of the population. It enhances accessibility, increases efficiency, strengthens clinical effectiveness, and improves user satisfaction. With the aim of improving quality of care, the MoHFW launched the National Quality Assurance Standards (NQAS) for District Hospitals in 2013 and subsequently for other levels of health facilities. These standards are accredited by ISQua (International Society for Quality in Healthcare) and are also recognized by IRDA and NHA.
As on 30th September 2022, a total of 3415 public health facilities have achieved National Quality Certification (1807 nationally certified which includes recertification also and 1608 at state level) ). In 2022, a total of 304 health facilities have been NQAS certified till 30th September 2022.The details are provided below:
| Level of facility | Number of facilities certified. |
| District Hospitals | 32 |
| Sub-Divisional Hospitals | 09 |
| Community health centres | 27 |
| Primary Health Centres | 182 |
| UPHC | 48 |
| Health & Wellness Centre – SHCs | 06 |
| Total | 304 |
2.12 National Urban Health Mission (NUHM)
National Urban Health Mission (NUHM) was approved on 1st May, 2013 as a sub-mission under an overarching National Health Mission (NHM), NRHM being the other sub-mission. NUHM envisages strengthening the primary health care delivery systems in urban areas and for providing equitable and quality primary health care services to the urban population with special focus on slum dwellers and vulnerable population. It also seeks to decongest secondary and tertiary health care facilities (District Hospitals/Sub-District Hospitals/Community Health Centre) by providing robust comprehensive Primary health care services in urban areas.
NUHM covers all cities and towns with more than 50,000 populations and district headquarters and State headquarters with more than 30,000 population. Also below UPHC, U-HWCs (Urban Health & Wellness Centers) on the population of 15,000-20,000 have been approved under 15th FC and PM-ABHIM. These U-HWCs are linked to the nearest UPHC –HWCs for administrative, financial, reporting, and supervisory purpose. The remaining cities/ towns are covered under National Rural Health Mission (NRHM). As part of Ayushman Bharat, the existing UPHCs are being strengthened as Health & Wellness Centres (HWCs) to provide preventive, promotive and curative services in cities closer to the communities.
Under NUHM, the Centre-State funding pattern is 60:40 for all the states w.e.f. FY 2015-16, except all North-Eastern states and other hilly States viz. Jammu & Kashmir, Himachal Pradesh and Uttarakhand, for which the Centre-State funding pattern is 90:10. In the case of UTs, from FY 2017-18, the funding pattern of UT of Delhi and Puducherry has been revised to 60:40 and rest of the UTs without legislature are fully funded by the Central Government.
Implementation of NUHM is through the State Health Department or the Urban Local Bodies (ULBs). In seven metropolitan cities, viz., Mumbai, New Delhi, Chennai, Kolkata, Hyderabad, Bengaluru and Ahmedabad the implementation is through the ULBs. For the other cities, the State Health Department decides whether the NUHM is to be implemented through them or the other urban local bodies. So far, 1162 cities have been covered under NUHM in 35 States/UTs.
Physical Progress:
The programme is being implemented in the States/UTs for more than 7 years period and accounts for presence of augmented infrastructure and human resources dedicated towards urban areas. According to the 1st Quarterly MIS Report i.e. for period April-June, 2022 submitted by the States/UTs, the information regarding progress of activities approved under NUHM is as follows: –
- Progress under Infrastructure
- 1162 cities/ towns covered under NUHM
- 5023 UPHCs & 202 UCHCs are functional
- 4698 UPHC-HWC are operationalised as per HWC portal (as on 26th December 2022)
- 1444 U-HWC are operationalised as per HWC portal (as on 26th December 2022)
- Progress Under HR under NUHM
- 4635 Medical Officers (3116 Full Time and 1519 Part-Time)
- 298 Specialists in-position
- 7785 Staff Nurse in-position
-
- 15183 ANMs in-position
- 3217 Pharmacist in-position
- 3330 Lab Technician in-position
- 429 Public Health Managers in-position
- 1192 Programme Management staff in-position at State/District/City level
- MHUs under NUHM
- 51 Mobile Health Units functional
- Progress under Community Process
- 72655 ASHAs are in-position. (One ASHA covers 200 to 500 households)
- 80978 Mahila Arogaya Samiti (MAS) are formed (One MAS covers 50- 100 households)
- As a part of Ayushman Bharat, the existing U-PHCs are being strengthened as Health & Wellness Centres (HWCs) to provide preventive, promotive and curative services in cities closer to the communities. So far, 4698 U-PHCs have been converted into AB-HWCs in the States/UTs (except Delhi). As per HWC portal data, about 2.4 Cr screenings done for Hypertension and around 1.8 Cr screenings done for Diabetes at these HWCs. Similarly, these functional AB-HWCs have done 73 Lakhs screening for oral cancer, 24.5 lakh for cervical cancer and 40.8 lakh for breast cancer in women as on 26.12.2022.
- National Quality Assurance Standards (NQAS) were developed for urban health facilities in Year 2016 and institutional framework has been set up in all States/UTs. Till date, 184 UPHCs have been quality certified at the National level and 177 UPHCs at the State level (As on 30th November 2022).
Financial Progress:
Since the launch of NUHM in FY 2013-14 till the FY 2021-22, funds to the tune of Rs. 8788.48 Crore and Rs.7165.87 Crore have been allocated and released respectively to the States/ UTs for implementation of the programme activities from FY 2022-23, all financial related matters have been merged with NHM.
2.13 Pradhan Mantri Ayushman Bharat Health Infrastructure Mission (PM-ABHIM):
Pradhan Mantri Atmanirbhar Swasth Bharat Yojana scheme (now renamed as Pradhan Mantri Ayushman Bharat Health Infrastructure Mission, PM-ABHIM) with an outlay of about Rs. 64,180 Cr over till FY 2025-26 was launched by Hon’ble Prime Minister on 25th October, 2021. This is the largest pan-India scheme for strengthening healthcare infrastructure across the country.
The measures under the scheme focus on developing capacities of health systems and institutions across the continuum of care at all levels viz. primary, secondary and tertiary and on preparing health systems in responding effectively to the current and future pandemics/disasters.
The Pradhan Mantri Ayushman Bharat Health Infrastructure Mission targets to build an IT enabled disease surveillance system by developing a network of surveillance laboratories at block, district, regional and national levels, in Metropolitan areas & strengthening health units at the Points of Entry, for effectively detecting, investigating, preventing, and combating Public Health Emergencies and Disease Outbreaks.
Increased investments are also targeted to support research on COVID-19 and other infectious diseases, including biomedical research to generate evidence to inform short-term and medium-term response to COVID-19 like pandemics and to develop core capacity to deliver the One Health Approach to prevent, detect, and respond to infectious disease outbreaks in animals and humans.
The main interventions under the ‘Pradhan Mantri Ayushman Bharat Health Infrastructure Mission’ scheme to be achieved by FY 2025-26 are:
Centrally Sponsored Components:
- Support for 17,788 rural Health and Wellness Centres in 10 High Focus States. Support for other States/UTs under XV Finance Commission Health Sector Grants and NHM.
- Establishing 11,024 urban Health and Wellness Centres in all the States.
- 3382 Block Public Health Units in11 High Focus states. Support for other States/UTs under XV Finance Commission Health Sector Grants and NHM.
- Setting up of Integrated Public Health Labs in all districts.
- Establishing Critical Care Hospital Blocks in all districts with population more than 5 lakhs.
Central Sector Components:
- 12 Central Institutions as training and mentoring sites with 150 bedded Critical Care Hospital Blocks.
- Strengthening of the National Centre for Disease Control (NCDC), 5 New Regional NCDCs and 20 metropolitan health surveillance units;
- Expansion of the Integrated Health Information Portal to all States/UTs to connect all public health labs;
- Operationalisation of 17 new Public Health Units and strengthening of 33 existing Public Health Units at Points of Entry, that is at 32 Airports, 11 Seaports and 7 land crossings;
- Setting up of 15 Health Emergency Operation Centres and 2 container based mobile hospitals; and
Setting up of a national institution for One Health, 4 New National Institutes for Virology, a Regional Research Platform for WHO South East Asia Region and 9 Biosafety Level III laboratories.
Progress so far:
- In the first year of support (2022-23), a total of 33 labs will be strengthened as per target. Fund release for 20 labs has been processed.
- Land allocation for new NIVs and One health Institute at Dibrugarh, Jabalpur, Jammu, Bangalore and Nagpur (One Health) has been completed. Contracts awarded to CPWD. Tenders in process.
- Building comprehensive surveillance system with more than 4000 labs.
- Digitisation of analytics, forecasting & early warning systems through the Integrated Health Information Platform (IHIP). It is designed to capture real-time, case-based epidemiological data of more than 33 plus health conditions.
- 37,000 new critical care beds with ICU & Oxygen.
- Health units at 50 International Points of Entry: 32 Airports, 11 Seaports & 7 land crossings.
- 3.Reproductive, Maternal, Newborn, Child, Adolescent Health Plus Nutrition (RMNCAH+N)
3.1 Immunization
- Pneumococcal Conjugate Vaccine (PCV) nationwide expansion: PCV was launched in phased manner in May 2017 and it was available in five States in the country till FY 2019-20. In FY 2021-22, in line with the Budget announcement 2021-2022, PCV has been expanded nationwide and is now available across all States/UTs. PCV has been expanded to all States/UTs across the country. Till October 2022, a total of 11.9 crore doses of PCV have been administered across the country since introduction.
- India’s FIC as per NFHS-5: NFHS 5 survey report has shown 14.4 percentage points increase in Full Immunization Coverage as compared to NFHS-4.
- Mission Indradhanush 4.0 was conducted from February 2022 to May 2022 vaccinating 59.9 lakh children and 15.1 lakh pregnant women
- India has committed to Measles and Rubella Elimination by 2023 and has achieved a Non Measles Non Rubella (NMNR) Discard Rate of 3.9/lakh population.
3.1.1 National COVID-19 Vaccination Programme:
On 16th January 2021, India launched the National COVID Vaccination Programme. COVID vaccination in the country commenced with vaccination to all Health Care Workers followed by Front line Workers, population aged ≥60 years and has subsequently expanded to cover the population aged 12 years and above. The Vaccination Programme is being guided by immaculate planning based on a regular review of scientific and global test practices by National Expert Group on Vaccine Administration for COVID-19 (NEGVAC).
Since the start of the COVID Vaccination drive, it has focused on taking decisions guided by science. Prioritizing our health workers, frontline workers and other vulnerable populations in a phased manner has been an excellent way to scale up the vaccination program.
Under the programme, all citizens irrespective of their income status are entitled to free vaccination. While those who have the ability to pay are encouraged to use private hospital’s vaccination centres.
Presently, several vaccines are being used in COVID Vaccination Drive, these include Serum Institute of India’s Covishield, Bharat Biotech’s Covaxin, ZyCoV-D (private hospitals only), World’s first plasmid DNA Vaccine, by Zydus Life Sciences, Corbevax, Nation’s first protein subunit vaccine by Biological E, COVOVAX SARS-CoV-2 rS Protein (COVID-19) recombinant spike protein Nanoparticle Vaccine of M/s Serum Institute of India Pvt. Ltd. (private hospitals only), GEMCOVAC-19, Nation’s first mRNA Vaccine by Gennova Biopharmaceuticals (private hospitals only) and the Russian Sputnik V(private hospitals only).
In just 9 months of the start of the COVID vaccination drive, India achieved a significant milestone of administering over 100 crore doses of COVID vaccines to its eligible adult population. India became one of the few countries to achieve this milestone. Subsequently, another set of 100 crores vaccine doses have been administered in the next 9 months, depicting sustainability.
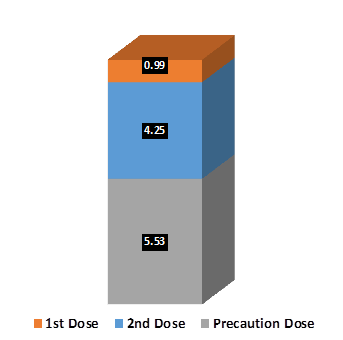
Out of the total eligible population, as on 6th December 2022, over 97% citizens have received 1st dose of COVID Vaccine while over 90% eligible citizens have received 2nd dose of the vaccine. Additionally, 22.30 crore precaution doses have also been administered across the country covering 27% of the eligible adult population.
3.1.2. Har Ghar Dastak
A nation-wide COVID-19 vaccination campaign Har Ghar Dastak was implemented from 3rd Nov till 31st Dec 2021 and from 1st June 2022 to 31st July 2022, which included awareness, mobilization and vaccination campaign through reaching out to all missed out and dropped out eligible beneficiaries through intensive rounds of House to House visit. Intensive rounds of door-to-door vaccination. It also focused on School-based vaccination campaign for children aged 12-18 years population along with emphasis on vaccination of beneficiaries in prisons, old-age homes, ashrams, mental health institutions etc.
The campaign aimed to ensure that all eligible beneficiaries are vaccinated with 1st dose, all due beneficiaries with 2nd dose and eligible for precaution dose of COVID-19 vaccines. The Ministry had created and shared an operational guideline that was shared with all State/UT.
With a special focus on the low performing districts, nodal officers (Joint Secretaries) were identified for regular follow ups and visits to the assigned states.
Further, Hon’ble Health & Family Welfare Minister also held an orientation session with NGOs & CSOs from across the country on the Har Ghar Dastak campaign. He discussed how an enhanced partnership between the government and these organizations would strengthen the campaign.
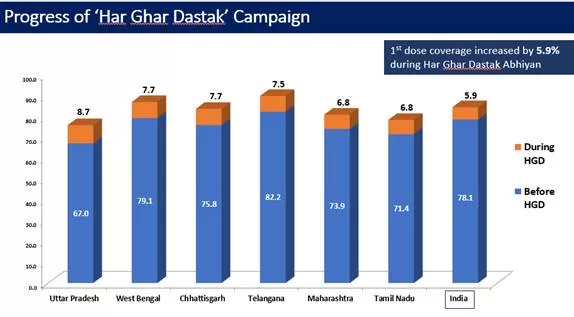
3.1.3. Achievement / Progress under Har Ghar Dastak Campaign:
Due to these efforts and sustained efforts by all States and UT, 1st dose coverage increased by 5.3% during Har Ghar Dastak Abhiyan (data till 30th Nov, 2021).
Similarly, the 2nd dose coverage increased by 11.7% during the campaign (data till 30th Nov, 2021).
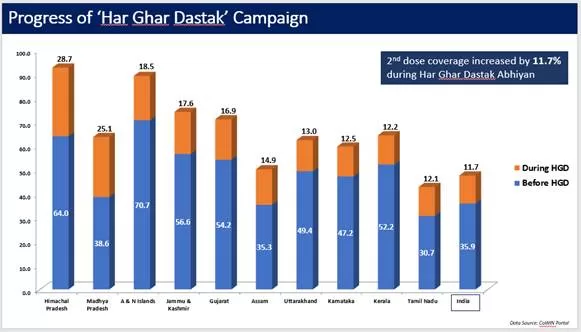
Achievement / Progress under Har Ghar Dastak 2.0 Campaign:
3.1.4. Best Practices under Har Ghar Dastak Campaign:
- Bihar: Ek Adhoora, Do se Poora Campaign – Mission 2nd Dose Campaign; Teeka Express; Teeka Wali Nav; and Motorbike Vaccination team.
- Himachal Pradesh: Suraksha ke rang naa honge pheeke jab samay par lagenge dono teeke initiative; Bulawa toli.
- Maharashtra: Committee of Sarpanch, Talathi, Gram Sevak, ASHA, AWW, Teachers for mobilization.
- Manipur: Religious Leaders Appealed for vaccination through recorded messages. These messages were shared through WhatsApp.
- Andhra Pradesh: Rewarding of best performing teams by District Civil Surgeon Kalaa Jathara, Dandora.
- Jharkhand: Nukkad Natak, Ramp & Wall writing.
3.1.5. Covid-19 Vaccination Amrit Mahotsava:
As part of the celebration of 75 years of India’s Independence, Azadi ka Amrit Mahotsav is being observed across the country. Under the aegis of ‘Azadi ka Amrit Mahotsava’, GoI launched the ‘COVID Vaccination Amrit Mahotsava’ initiative from 15th July 2022 to 30th September 2022 under which free precaution doses were administered to all eligible beneficiaries (persons aged 18 years & above who have completed 6 months or 26 weeks after the 2nd dose) at all Government CVCs. A camp-based strategy was implemented wherein special vaccination sessions were organized at various places such as public & private offices, schools, colleges, routes of religious yatras, industrial establishments etc.
During the initiative, more than 76.56 lakh first dose, 2.37 crore second dose and 16.07 crore precaution dose have been administered. More than 24.94 lakh doses per day have been administered including 20.87 lakh doses precaution dose per day. Further, a total of 13,01,778 such camps have been organized for free covid vaccination.
As on 15th July 2022, only 8% of the eligible population aged 18 years and above had received the precaution dose. However, with the help of this initiative, 27% of the eligible population has received their precaution dose.
3.2 Maternal Health
As per the Special Bulletin on MMR released by the Registrar General of India (RGI), the Maternal Mortality Ratio (MMR) of India has improved further by a spectacular 6 points and now stands at 97/ lakh live births. The Maternal Mortality Ratio (MMR) is defined as the number of maternal deaths during a given time period per 100,000 live births.
As per the statistics derived from Sample Registration System (SRS), the country has witnessed a progressive reduction in MMR from 130 in 2014-2016, 122 in 2015-17, 113 in 2016-18, 103 in 2017-19 and to 97 in 2018-20.
Upon achieving this, India has accomplished the National Health Policy (NHP) target for MMR of less than 100/lakh live births and is on the right track to achieve the SDG target of MMR less than 70/ lakh live births by 2030.
The outstanding progress made in terms of the number of states which have achieved Sustainable Development Goal (SDG) target, the number has now risen from six to eight leading with Kerala (19), followed by Maharashtra (33), then Telangana (43) and Andhra Pradesh (45), subsequently Tamil Nadu (54), Jharkhand (56), Gujarat (57) and lastly Karnataka (69).
- a)Key highlights of NFHS-5 (2019-21)-Maternal Health:
- 1stTrimester ANC Registration increased from 58.6% (NFHS-4) to 70% in NFHS-5
- Institutional Deliveries increased from 78.9% (NFHS-4) to 88.6% in NFHS-5
- Skilled Birth Attendant (SBA) attended deliveries increased from 81.4% (NFHS-4) to 89.4% in NFHS-5.
- b)Surakshit Matritva Aashwasan (SUMAN):It aims to provide assured, dignified, respectful and quality healthcare at no cost and zero tolerance for denial of services for every woman and newborn visiting the public health facility to end all preventable maternal and newborn deaths. Till 14th December 2021, 10,010 facilities have been notified under SUMAN.
- c)Maternal Perinatal Child Death Surveillance Response (MPCDSR)software was launched by the Hon’ble Union health Minister of Health & Family Welfare in September 2021. This was followed by the National ToT of the software in October 2021.
- d) Midwifery Educator Training: The Government of India has taken a policy decision to roll out Midwifery Services in the country in order to improve the quality of care and ensure respectful care to pregnant women and newborns. “Guidelines on Midwifery Services in India, 2018” was released during the Partners Forum held in December 2018 at New Delhi.
- Resumption of Midwifery training: Training of Midwifery Educators (MEs) was halted due to the pandemic, which was resumed in September 2021 at NMTI in Telangana.
- Release of Scope of Practice: “Scope of Practice document for Midwifery Educators (ME) and Nurse Practitioner Midwife (NPM)” has been released in collaboration with the Indian Nursing Council (INC). It acts as a guiding document for their education, regulation and ongoing professional development.
- e)Pradhan Mantri Surakshit Matritva Abhiyan (PMSMA): Since inception, more than 3.02 crore antenatal check-ups have been conducted and 25.46 lakh high risk pregnancies have been identified under PMSMA across States/ UTs till 4th December 2021.
- f)LaQshya:It aims to improve the quality of care in Labour Room and Maternity Operation Theatres to ensure that pregnant women receive respectful and quality care during delivery and immediate postpartum. Till 30th November 2021, 421 Labour Rooms and 350 Maternity Operation Theatres are LaQshya certified at national level. During the FY 2021-22, 99 Labour Rooms and 79 Maternity Operation Theatres are LaQshya certified at national level.
- g) Janani Suraksha Yojana (JSY): 36.38 lakhs beneficiaries received benefits under JSY during the period of April-September 2021 (Provisional data, 2021-22).
- h)Ensuring Maternal health services during Covid-19 pandemic: On 19th May 2021, a webinar was conducted on ‘Ensuring Maternal Health Services in COVID-19 Pandemic’ with support from domain experts and some State’s maternal health nodal officers with the objective to re-emphasize and reinforce MoHFW’s guidance on essential maternal health services during COVID-19 pandemic and also to impart standardized and updated knowledge on management of COVID-19 during different stages of pregnancy and to disseminate good practices from the States and medical colleges.
- i)Guidelines released:
- Collaborative framework for management of tuberculosis in pregnant women was released to help the States / UTs, Mission Directors and programme officers to ensure early detection and timely management of TB cases in pregnant women in India. National training has been planned for FY 2021-22.
- Standard Operating Procedures for HIV & Syphilis Screening of Pregnant Women at VHSND Sites was released to define the standard operating procedures for implementation of HIV & Syphilis screening at VHSND sites.
- Guidelines on operationalisation of maternal health services during COVID-19 pandemic’ was finalised and released in September 2021.
- j) Comprehensive Abortion Care (CAC): More than 16,000 Medical Officers have been trained in CAC trainings up to December, 2021. Virtual training of trainers (ToT) on CAC has been conducted for 17 States and 328 Master Trainers have been trained till December 2021.
- k) Medical Termination of Pregnancy (Amendment) Act & Rules 2021: The MTP Act recognized the importance of providing safe, affordable, accessible and legal abortion services to woman who needs to terminate a pregnancy due to certain therapeutic, eugenic, humanitarian or social grounds. The Act was amended for expanding base of beneficiaries to provide safe abortion services.
The Medical Termination of Pregnancy (Amendment) Act, 2021 was published in the Gazette on 25th March 2021 and followed by its notification for commencement on 24th September, 2021. The Rules were formed and notified for commencement on 12th October 2021.
The amended MTP Act is a step towards safety and well-being of women and will enlarge the ambit and access of women to safe and legal abortion without compromising on safety and quality of care.
The Medical Termination of Pregnancy (Amendment) Act, 2021 has introduced the following changes in The MTP Act 1971:
- Requirement of opinion of one registered medical practitioner for termination of pregnancy up to twenty weeks of gestation
- Requirement of opinion of two registered medical practitioners for termination of pregnancy of twenty to twenty-four weeks of gestation
- Enhanced the upper gestation limit from twenty to twenty-four weeks for such category of woman as may be prescribed by rules in this behalf
- Non-applicability of the provisions relating to the length of pregnancy in cases where the termination of pregnancy is necessitated by the diagnosis of any of the substantial foetal abnormalities diagnosed by a Medical Board
- Strengthening protection of privacy of a woman whose pregnancy has been terminated
- Failure of contraceptive clauses extended to women and their partner.
3.3 Child Health
- a) As per the latest report of Sample Registration System (SRS) released in October 2021 by the Registrar General of India (RGI), Infant Mortality Ratio (IMR) of India has declined from 32 per 1000 live births for the year 2018 to 30 per 1000 live births for the year 2019.
27 States/ UTs namely Mizoram, Nagaland, Sikkim, Kerala, A & N Islands, Goa, Lakshadweep, Puducherry, Manipur, Delhi, D & N Haveli, Chandigarh, Tamil Nadu, Maharashtra, Daman & Diu, Punjab, Himachal Pradesh, Jammu & Kashmir including Ladakh, West Bengal, Karnataka, Tripura, Telangana, Andhra Pradesh, Gujarat, Haryana, Jharkhand, Uttarakhand have achieved National Health Policy Target (28 per 1000 live births by 2019).
- b)Facility Based Newborn Care (FBNC) program: 914 Special Newborn Care Units (SNCUs) at District/ Medical College Level and 2,579 Newborn Stabilization Units (NBSUs) at the level of FRUs/ CHC levels are functional to provide services to sick and small newborns. A total of 7.53 lakhs newborns received treatment in Special Newborn Care Units (SNCUs) at District Hospitals and Medical Colleges (April-November, 2021).
- c)National Newborn Weekis observed from 15th to 21st November every year to reinforce the importance of newborn health as a key priority area and reiterates its commitment at the highest level. In the year 2021 also, a virtual event for the National Newborn Week was organized by MoHFW on 15th November 2021. The theme of National Newborn Week for this year is “Safety, Quality and Nurturing care – Birth Right of Every Newborn”. National Newborn Week and SAANS Campaign IEC posters were also released by MoHFW on this day for dissemination of information and for triggering behaviour change and demand generation on newborn health.
- d)MusQan – Quality improvement initiative of Child Health services:The Hon’ble Union Minister of Health and Family Welfare launched “MusQan” initiative on 17th September 2021 for ensuring child friendly services in Public Health facilities on the occasion of World Patient Safety Day. The initiative will be focusing on improving the quality parameters for ensuring safety and availability of infrastructure, equipment, supplies, skilled human resources, clinical protocols, evidence based practices etc. at public health facilities. National dissemination of “MusQan – Quality improvement initiative of Child Health services” was conducted on 3rd December 2021.
- e)Home Based Newborn Care (HBNC) program: A total of 98.63 lakh newborns received complete schedules of home visits by ASHAs whereas 3.6 lakhs identified sick newborns were referred to health facilities by ASHAs during the period of January-September 2021.
- f) Home Based Care of Young Child (HBYC): In FY 2021-22, approval has been accorded for 604 Districts including all Aspirational Districts to implement HBYC across States/UTs except Goa. More than 1.2 crores home visits conducted to young children (3 months-15 months) by ASHAs during January-September, 2021. In addition, a supportive supervision handbook for ASHA Facilitators and ANM/MPW on HBNC and HBYC programs has been shared with all States/UTs aiming to improve on job mentoring and supervision by AF/ANM/MPW to ensure quality home visits by ASHAs.
- g)UnderIntensified Diarrhoea Control Fortnight (IDCF), 2021, approximately 8 crore children up to five years of age were provided with ORS and Zinc against the target of 13.37 crore children of the same age group. The data compilation for the IDCF/Diarrhoea prevention activities for the year 2021 round is in process.
- h)National Deworming Day (NDD): During 12thround of NDD conducted in February 2021, around 17.75 crore children in the age group of 1-19 years had been provided Albendazole tablets against the target of 20.94 crore children of the same age group. The 13th round of NDD is being implemented in 34 States / UTs during the period of August-November, 2021.
- i) Nutrition Rehabilitation Centres (NRCs): Nearly 1.04 Lakhs Severe Acute Malnutrition (SAM) children with medical complications received treatment at 1073 Nutrition Rehabilitation Centres during FY 2020-21. During FY 2021-22 (April-September 2021), 59,424 Severe Acute Malnutrition (SAM) children with medical complications received treatment at 1080 NRCs.
- j)Lactation Management Centres (LMCs):As of FY 2020-21, 15 CLMCs and 3 LMUs are established in 7 States (Maharashtra, West Bengal, Goa, Gujarat, Madhya Pradesh, Tamil Nadu and Uttar Pradesh).
- k)Anemia Mukt Bharat (AMB) program(April-September, 2021)
-
- 2.0 crore children of age group 6-59 months were provided 8-10 doses of Iron Folic Acid (IFA) Syrup every month
- 1.9 crore children of age group 5-9 years were provided 4-5 IFA Pink tablets every month
- 3 crore adolescent of age group 10-19 years provided 4-5 IFA Blue tablets every month
- 1.3 crore pregnant women and 0.6 crore lactating women were provided 180 IFA Red tablets.
- l)Rashtriya Bal Swasthya Karyakram (RBSK): During FY 2021-22, due to COVID- 19 pandemic, community level screening activities by Mobile Health Teams of RBSK got affected. As reported by States/UTs in HMIS during April-November, 2021, 4.2 crores children have been screened by Mobile Health Teams. 1.11 crores newborn have been screened at Delivery points under RBSK Program during April-November, 2021.
- m)Social Awareness and Actions to Neutralize Pneumonia Successfully (SAANS): SAANS Campaign has been rolled-out in the States/ UTs from 12thNovember, 2021 – 28th February 2022 with the aim to accelerate the action against Childhood Pneumonia by generating awareness around protect, prevent and treatment aspects of Childhood Pneumonia and to enhance early identification and care seeking behaviours among parents and caregivers. Additionally, awareness generation, promotion and administration of Pneumococcal Vaccine (PCV) has also been included under SAANS campaign for the year 2021.
- n)India COVID-19 Emergency Response and Health Systems Preparedness Package (Phase II):Under India COVID-19 Emergency Response and Health Systems Preparedness Package (Phase II), the focus has been given on strengthening of Paediatric Care Facilities at Medical College, District Hospital and Sub-District level facilities. As part of ECRP-II, support has been provided for Paediatric ICU beds, Paediatric HDU beds and Paediatric Oxygen supported beds under dedicated COVID Care Unit at District level. Also, augmentation of Paediatric ICU beds at various levels of facilities has been supported.
- o) “Guidelines on Operationalization of COVID-19 Care Services for Children & Adolescents” was released on 14thJune 2021 and “Guidelines for Management of COVID 19 in Children (below 18 years)” on 18thJune 2021. The Guidelines focus on all aspects of Paediatric Care which includes additional bed capacity for paediatric care during the peak daily cases considering projections for paediatric cases and admissions at different level of facilities; Augmentation of facilities – requirement of drugs, equipment, consumables, Human Resources, capacity building etc; Designating specific areas in the COVID facilities for paediatric care and accompanying parents’/ family members of child; Facility and community level planning; Transport linkages; Management of CoVID in the community settings; IEC Plan; Governance mechanism etc.
3.4 Family Planning
- a) Key highlights of NFHS-5 (2019-21):
- India has achieved a replacement level of Total Fertility Rate (TFR)It is currently at 2.0 . 31 States/UTs have achieved replacement level of TFR.
- Total unmet need has reduced substantially to 9.4% (NFHS-5) from 12.9% (NFHS-4)
- Use of modern contraceptives has increased substantially.
- IUCD use has shown an increase for the first time since NFHS-1. It has increased by 0.6% points, from 1.5 % in NFHS-4 to 2.1 % in NFHS-5.
- 29 States have >70% eligible couples in need of contraception (as against 12 States in NFHS 4). It shows that Family Planning demand generation activities have shown a positive result.
- Overall positive shift towards spacing methods (increase in all spacing methods).
- b) The performance of family planning services in FY 2022-23 (up to September, 2022) is as follows:
- Total Sterilization: 11.43 Lakhs
- Post-partum IUCD (PPIUCD): 15.99 lakh
- PPIUCD acceptance rate (%) in public health facilities: 25.3 %.
- Contraceptive Injectable MPA (Antara Program): 16.51 lakh doses have been administered
- Non-hormonal Pill Centchroman (Chhaya): 52.25 lakh strips of Centchroman(Chhaya)
- c)Mission Parivar Vikas (MPV) – Mission Parivar Vikas (MPV) – MPV was launched in November, 2016 to substantially increase access to contraceptives and family planning services in 146 High Fertility Districts in seven high focus States with a Total Fertility Rate (TFR) of 3 and above. These Districts are from the States of Uttar Pradesh (57), Bihar (37), Rajasthan (14), Madhya Pradesh (25), Chhattisgarh (2), Jharkhand (9) and Assam (2).
MPV has been expanded to the remaining Districts of seven high focus States and six North Eastern States (Arunachal Pradesh, Manipur, Meghalaya, Tripura, Nagaland and Mizoram) in October, 2021.
MPV Districts have shown substantial increase in improving access to contraceptives.
The performance family planning services of in MPV in 13 MPV states in FY 2022-23 (Up to September,2022) is as follows:
- Total number of Sterilizations: 3.36 lakh sterilization
- Post-partum IUCD (PPIUCD): 8.61 lakh
- PPIUCD acceptance rate (%) in public health facilities: 18.65 %
- Contraceptive Injectable MPA (Antara Program): 11.06 lakh doses
- Non-hormonal Pill Centchroman (Chhaya): 34.41 lakh strips of Centchroman (Chhaya)
3.5 Rashtriya Kishor Swasthya Karyakram (RKSK)
- a)Adolescent Friendly Health Clinics (AFHCs):38 lakh adolescents received counselling and clinical services at Adolescent Friendly Health Clinics (AFHCs).
- b) Weekly Iron Folic Acid Supplementation (WIFS): 3 crore adolescents had been provided Weekly Iron Folic Acid Supplementation (WIFS) every month besides Nutrition Health Education till November 2021.
- c)Peer Educator program: Significant progress has been made in implementation of Peer Educator program with selection of 1.69 lakhs Peer Educators in FY 2021-22 (upto September 2021) to cover for those who have left, grown up or selected fresh in the newer selected districts
- d) Adolescent Health Days (AHDs): 64,577 Adolescent Health Days (AHDs), quarterly community & school level activities were conducted till September 2021 to create awareness about adolescent health issues.
- e)Ayushman Bharat School Health and Wellness:
- School Health & Wellness Programme (launched in February 2020) is being implemented in government and government aided schools in Districts (including most of the Aspirational Districts) of the country in the first phase of the implementation.
- Two teachers, preferably one male and one female, in every school, designated as “Health and Wellness Ambassadors” (HWAs) shall be trained to transact health promotion and disease prevention information on 11 thematic areas in the form of interesting joyful activities for one hour every week.
- The States have initiated the Health and Wellness Ambassadors training.
- Till 30thNovember 2021, 1.29 Lakh HWAs have been trained and about 67,391 principals are oriented under the Programme. HWA sessions are gradually starting in the States with reopening of schools.
- f)NFHS-5 (2019-21) key highlights:
- 32 States/UTs have shown reduction in early marriages and 25 have shown reduction in prevalence of teenage pregnancies as compared to NFHS-4.
- NFHS-5 (2019-21) has reflected that women aged 15-24 yrs who use hygienic methods of protection during their menstrual period have increased to 77.3% from 57.6% (NFHS-4). 35 out of 36 States/ UTs have shown significant improvement in use of hygienic methods during menstruation.
3.6 Pre-Conception and Pre-Natal Diagnostic Techniques (PC & PNDT):
- As per Quarterly Progress Report (QPR) of June 2021, submitted by the States/UTs, total 72,965 Diagnostic facilities have been registered under the PC& PNDT Act. So far, a total of 2589 machines have been sealed and seized for the violations of the law. A total of 3,201 court cases have been filed by the District Appropriate Authorities under the Act and 617 convictions have so far been secured, leading to suspension / cancellation of medical licenses of 145 doctors.
- NFHS-5 (2019-21) has also recorded improvement of 10 points in the sex ratio at birth at the national level from 919 in NFHS-4 to 929. 23 States/UTs have shown improvement whereas 13 States/UTs show decline in sex ratio at birth.
- Review meetings were conducted in all 36 States/UTs and implementation of PC&PNDT Act was reviewed in all aspects.
- Capacity building of District Appropriate Authorities and PNDT Nodal Officers was conducted in the State of Bihar, Telangana and Andhra Pradesh.
- Training of public prosecutors was organized in Chhattisgarh and Telangana.
- 4.NATIONAL TUBERCULOSIS ELIMINATION PROGRAMME
Performance 2014-2022
With the target of achieving Sustainable Development Goals related to TB by 2025, five years ahead of the global targets of 2030, the Ministry implements National TB Elimination Programme with the following objectives: –
- Early diagnosis of TB patients, prompt treatment with quality assured drugs and treatment regimens.
- Engaging with the patients seeking care in the private sector.
- Prevention strategies including active case finding and contact tracing in high risk /vulnerable population.
- Airborne infection control.
- Multi-sectoral response for addressing social determinants.
Achievements in Key Programme Indicators over last 9 years are as under:
- TB Notification: The overall notification of TB cases has improved by 55% over the last 6 years. The missing TB cases had reduced from 10 lakhs in 2017 to 2.4 lakhs in 2019. In 2020, however, there has been a 25% reduction in TB case notification due to the COVID pandemic. In 2021, India was able to recover from the pandemic by notifying 18% more TB cases as compared to 2020. India could notify 18.41 lakhs TB cases in Jan-Sep 2022 which if annualized would surpass the pre-COVID level of 2019.
- Private Sector Notification: With a focused and targeted engagement with the private sector through interventions like Patient Provider Support Agency (PPSA), gazette notification for mandatory notification of TB cases, incentives for notification of cases and collaborations with professional bodies like IMA, IAP, FOGSI , there has been an increase in private sector notification by more than 6 times over the past 8 years (from 1.06 lakh cases in 2014 to 6.65 lakhs in 2019 and 5.57 lakhs in 2020). In 2021, the country was able to notify 6.89 lakhs TB cases (Highest ever Private sector notification) accounting to 32% of total notification. In 2020 (Jan-June), 3.64 lakh cases have already been notified from the private sector. In 2022, Jan-Sep India was able to notify 5.46 lakhs of TB cases from Private sector.
- Introduction of newer anti-TB drug – Bedaquiline, Delaminid: Bedaquiline containing DRTB regimens had been rolled out pan-India across all states and UT’s. This drug has been given to multi drug resistant TB patients with or without resistance to fluoroquinolones. Between Jan-Sep 2022, a total of 16338 patients were initiated on Longer oral M/XDR-TB regimen and 25960 patients were initiated on Shorter MDR/RR-TB regimen (Oral/ Inj).
- TB Treatment Success Rate: Over the last 8 years, in spite one third of notification coming from the private sector, the programme was able to sustain the treatment success rate of above 80%. In 2021, the success rate had reached to 84% and in 2022 (Jan-Sep) it further improved to 84.2%.
- Nikshay Poshan Yojana: Undernutrition being an important risk factor for TB, the Government introduced a scheme of Nikshay Poshan Yojana (NPY) in April 2018 for providing Rs 500/month as DBT to support nutrition of TB patients for the entire duration of treatment. Cumulatively, till, 30th Sep 2022, NTEP has disbursed Rs 1899 Cr to 71.93 lakhs TB patients.
- Active Case Finding: For reaching out to missing TB patients, the Government begun systematic active TB case finding in high-risk groups. The programme has proactively conducted house to house search of TB cases among these vulnerable population. This includes people living with HIV, diabetics, undernourished, residential institutes like prison, asylums, old age homes, orphanages, tribal area, marginalized population. This activity has resulted in the diagnosis of an additional 2.52 lakhs TB cases over the past 4 years.
- TB-HIV collaborative services: HIV and TB care services have been now made accessible from ART centre. In addition to ease of access, the interventions at ART centres across the country incorporate comprehensive package of services to reduce the burden of TB among People living with HIV AIDS (PLHA). These include rapid diagnosis of TB with CBNAAT, Fixed Dose Combination daily treatment for HIV & TB for HIV-TB co-infected patients, ICT based adherence support (99DOTS), INH Preventive Therapy for PLHIV (IPT), Infection Control Measures and Adverse Drug Reactions (ADR) monitoring system. In the year 2021, 38440 TB HIV patients were diagnosed. out of which 35763 (93%) were put on ART. In the year 2022 (Jan-Sep), 30950 TB-HIV patients were diagnosed, out of which 23057 (64.5%) were put on ART.
- Infrastructure Scale Up: There had been a huge infrastructure scale up of TB laboratory services. Designated Microscopy Centres (DMCs) have been increased by 61% (13583 in 2014 to 23028 in 2022) over the past 9 years and 4720 new Molecular diagnostic laboratories have been established over the last 8 years.
- State/UTs commitment To End TB by 2025: 26 State/UTs have committed publicly to End TB on or before 2025. (Andaman & Nicobar Islands; Arunachal Pradesh; Assam; Chandigarh; Chhattisgarh; DNHⅅ Gujarat; Himachal Pradesh; Haryana; Karnataka; Kerala; Ladakh; Lakshadweep; Madhya Pradesh; Meghalaya; Mizoram; Punjab; Sikkim; Tamil Nadu; Tripura; West Bengal; Manipur; Uttar Pradesh; Jharkhand; Puducherry; Rajasthan)
- Sub National disease-free certification: To monitor the trends of TB Epidemic at State/UTs/District level, the ministry has introduced a novel initiative of estimating disease burden through a methodology of community level survey (Inverse sampling methodology) and tracking drug sales data in the private sector and measuring the level of under-reporting to the programme. Through this methodology State/UTs/District level estimates of TB disease is derived and measured against the baseline of 2015.
In the year 2020, the State of Kerala, UTs of Lakshadweep, Puducherry and 35 districts have successfully achieved various level of reduction in TB incidence. The UT of Lakshadweep and the district of Budgam in J&K were declared as the first UT & the first district in the country to achieve more than 80% reduction of TB incidence. (SDG Targets).
In 2021, 3 States (Kerala, DNHDD & Puducherry) received Silver (>40% reduction) & 5 States (Gujarat, Himachal Pradesh, Sikkim, Tripura, Ladakh) received Bronze (>20% reduction). Whereas, 8 districts receive Gold (>60% reduction), 27 districts received Silver & 56 districts received bronze.
- Pradhan Mantri TB Mukt Bharat Abhiyaan: Pradhan Mantri TB Mukt Bharat Abhiyaan was launched by Honorable President of India with the objectives to provide additional patient support to improve treatment outcome of TB patients, augment community involvement and leverage Corporate Social Responsibility (CSR) activities.
Achievements under PMTBMBA (17.11.2022):
- Ni-kshay Mitra registered: 46429
- TB patients on treatment: 13.30 lakhs
- TB patients consented to receive community support: 10.28 lakhs
- Commitment by Ni-kshay Mitra for TB patients: 10.22 lakhs
Summary of achievements:
| Indicators | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022* | ||
| TB Notification (Lakhs) | 15.5 | 16.08 | 17.55 | 18.28 | 21.56 | 24.04 | 18.05 | 21.35 | 18.41 | ||
| TB Notification-Private Sector (Lakhs) | 1.06 | 1.84 | 3.3 | 3.83 | 5.42 | 6.78 | 5.59 | 6.89 | 5.46 | ||
| TB Treatment Success Rate | 81% | 87% | 78% | 79% | 81% | 81% | 82% | 84% | 84.2% | ||
| Nikshay Poshan Yojana-DBT (Lakhs) (Beneficiaries paid at least one benefit) |
– | – | – | – | 12.92 | 15.92 | 11.66 | 13.26 | 11.10 | ||
| Active Case Finding
(Additional Cases diagnosed) |
– | – | – | – | 47307 | 62958 | 52273 | 73772 | 19449 | ||
| Infrastructure | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022* | ||
| Designated Microscopy Centres | 13583 | 13886 | 13888 | 15307 | 16212 | 20356 | 21717 | 21820 | 23028 | ||
| Cartridge based Nucleic Acid Amplification Test (CBNAAT)/Truenat | 40 | 80 | 121 | 628 | 651 | 1135 | 3147 | 3760 | 4760 | ||
*Jan – Sep 2022
- National Programme for Tobacco Control and Drug Addiction Treatment [NPTCDAT]
- Continuous steps are being taken to increase awareness on ill-effects of tobacco use. Under the National Tobacco Control Programme, digitization has been encouraged and an online portal/Management of Informatics System has been developed for the States to provide online reporting of the activities down from the district level. States too are appreciating the importance of the Online reporting / real time data and are participating in this wholeheartedly.
- India has been committed towards tobacco control. Taking forward the agenda and pursuing the international commitments, India assumed the Presidency of the Meeting of Parties Bureau, to support Parties (countries) to implement the Protocol to Eliminate Illicit Trade in Tobacco Products.
- The Ministry under the Cigarettes and Other Tobacco Products (Prohibition of Advertisement and Regulation of Trade and Commerce, Production, Supply and Distribution) Act, 2003 (COTPA 2003) has notified the new specified health warnings to be displayed on all tobacco product packs, which came into effect on 1st December, 2022. The Image 1 will be effective from December 2022 and Image 2 will be displayed from 1st December 2023. The graphic health warnings are a potent tool to create awareness about the serious and adverse health consequences of tobacco usage especially among the youth, children and illiterate persons.
| Image 1 | Image 2 | ||
 |
 |
 |
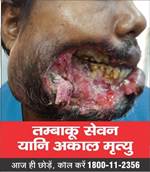 |
| 1st December, 2022 – 30th November, 2023 | 1st December, 2023 – 30th November, 2024 | ||
- Non Nicotine Replacement Therapy (NRT): The Non Nicotine Replacement Therapy (NRT) has been included in the National List of Essential Medicine (NLEM), 2022. This will facilitate the States to procure the NRT through National Health Mission and hence the availability of the drug would be more to the potential clients/patients.
- Substance Use Disorders (SUDs) includes a spectrum of problems caused by the persistent misuse of mind altering substances, and can range from a harmful use to dependence. Considering the importance that physicians are able to effectively identify, diagnose and manage the problems of substance use disorder, the “Standard Treatment Guidelines for the Management of Substance Use Disorders and Behavioural Addictions” was released. These Guidelines have been developed as a resource material for the general physicians in primary care setting to provide them necessary know-how for assessment and management of these disorders. Pocket book on Standard Treatment Guidelines for Substance Use Disorders and Behavioural Addictions and Mobile APP (Android & iOS) “Addiction-Rx” has also been developed for assisting physicians to provide quality care in substance use disorders, under Drug De-Addiction Programme
- Pradhan MantriSwasthya Suraksha Yojana (PMSSY)
- The Pradhan MantriSwasthya Suraksha Yojana (PMSSY) envisages creation of tertiary healthcare capacity in medical education, research and clinical care, in the underserved areas of the country. It aims at correcting regional imbalances in the availability of affordable/reliable tertiary healthcare services and also augmenting facilities for quality medical education in the country. The scheme has two broad components:
(i)Setting up of All India Institute of Medical Sciences (AIIMS);
(ii)Up-gradation of existing Government Medical Colleges/Institutions (GMCIs).
- So far, establishment of 22 new AIIMS and 75 up-gradation Projects of existing Government Medical Colleges/Institutions (GMCIs) have been approved under this scheme.
6.1 Six AIIMS under Phase-I:
- Six AIIMS approved under Phase- I (AIIMS-Bhopal, AIIMS-Bhubaneswar, AIIMS-Jodhpur, AIIMS-Patna, AIIMS-Raipur and AIIMS- Rishikesh) are already fully functional. All key hospital facilities and services such as OPD, IPD, Emergency, Trauma, Blood Bank, ICU, Diagnostic and Pathology are functioning.
- The total bed capacity of 6 AIIMS is at present 5764 against the sanctioned bed capacity of 5760.
- There are 612 PG seats and 750 MBBS seats in 6 AIIMS for Academic Year 2022-23.
6.2 New AIIMS under Phase-II, IV, V, VI & VII:
- 16 AIIMS have been sanctioned/approved by the Cabinet in subsequent phases. Following facilities and services have been made functional in these institutes:
- MBBS classes and OPD services started at following 10 AIIMS at Gorakhpur (UP) ,Raebareli (UP), Nagpur (Maharashtra), Kalyani (West Bengal), Mangalagiri (Andhra Pradesh), Bibinagar (Telangana), Bathinda (Punjab) Deoghar (Jharkhand), Bilaspur (Himachal Pradesh) and Rajkot (Gujarat). Of these, IPD facilities on a limited scale have been started in 9 AIIMS at Raebareli, Mangalagiri, Nagpur, Kalyani, Gorakhpur, Bathinda, Bilaspur, Deoghar and Bibinagar. in the current financial year 3550 hospital beds functional in these 9 AIIMS.
- MBBS classes have started in AIIMS at Guwahati (Assam) ,Vijaypur (Jammu), and Madurai (Tamil Nadu).
- There are 131 PG seats in 6 AIIMS (Mangalagiri, Nagpur, Bibinagar, Bathinda, Raebareli, Deoghar) and intake of 1285 MBBS students in 13 AIIMS (Mangalagiri, Nagpur, Kalyani, Gorakhpur, Bathinda, Raebareli, Deoghar, Bibinagar, Guwahati, Bilaspur, Vijaypur, Rajkot and Madurai) for Academic Year 2022-23.
6.3 Up-gradation of existing Government Medical Colleges /Institutes:
- The Up-gradation programme broadly envisages improving tertiary health infrastructure through construction of Super Speciality Blocks / Trauma Care Centres etc. and/or procurement of medical equipment at/for existing Government Medical Colleges / Institution.
- Since inception of the Scheme, 60 upgradation projects of existing Government Medical Colleges / Institutions have been completed, adding about 13982 Super-specialty beds including 2397 ICU beds. The Super Specialty Blocks /Trauma Centres constructed in these upgradation projects are also being used as COVID Hospital Blocks. The civil constructions of the following 6 projects have been completed during 2022-23 (upto November, 2022):
| S. No. | Name of the GMC/ Institute |
Name of the State | Phase | Type of facility | Total Beds | ICU Beds | No. of Super Specialties |
| 1 | Indira Gandhi Medical College, Shimla | Himachal Pradesh | III | SSB | 283 | 53 | 10 |
| 2 | TD Medical College, Alappuzha | Kerala | III | SSB | 279 | 62 | 9 |
| 3 | North Bengal Medical College, Siliguri, Darjeeling | West Bengal | III | SSB | 255 | 89 | 8 |
| 4 | GMC, Kanpur | Uttar Pradesh | IV | SSB | 270 | 30 | 12 |
| 5 | GMC, Jaipur | Rajasthan | IV | SSB | 231 | 51 | 5 |
| 6 | GMC, Bhavnagar | Gujarat | IV | SSB | 193 | 47 | 6 |
Setting up of Critical Care Hospital Blocks:
- In order to avoid mixing of COVID and non COVID patients, at many places full hospitals were required to be designated as COVID dedicated facilities. To control spread of the infectious disease, The PM-ABHIM Scheme envisages establishment of 150 bedded Critical Care Hospital Blocks (CCHB) in 12 Central Hospitals under the Central Sector Component at a total cost of Rs. 2220 crores. These institutes include – AIIMS at Delhi, Bhopal, Bhubaneswar, Jodhpur, Patna, Raipur and Rishikesh, PGIMER at Chandigarh, JIPMER at Puducherry, RIMS at Imphal, NEIGRIHMS at Shillong and IMS of BHU at Varanasi.
- The allocation under the scheme broadly translates to Rs. 120 crores as capital cost which includes civil construction, ICUs, HDUs, Dialysis, OTs, MGPS, CSSD, Hospital Beds and furniture and medical equipment. In addition, estimated operating expenses for salary of doctors and paramedics are Rs. 28.0 crore per annum and Rs. 12.0 crore per annum for other operating expenses on running the hospital services. The recurring cost will however be available after CCHB becomes functional and only upto 2025-26, after which Institutes will be expected to meet this requirement out of their regular budget.
- Medical Education
- The historic National Medical Commission Act was passed by the Parliament in August, 2019. Now, the National Medical Commission has been constituted with effect from 25th September, 2020 and the years old MCI has been dissolved and the Indian Medical Council Act, 1956 has been repealed. The principal change in the regulatory mechanism is that the regulator will be primarily ‘selected’ rather than ‘elected’. The National Medical Commission will steer the reforms in medical education. This will include increase in UG & PG seats along with improved access to quality and affordable medical education and maintaining high ethical standards in medical profession. Some of the key areas in which NMC will work include – implementation of National Exit Test (NEXT) for the medical graduates, guidelines for determination of fee for 50% seats in private medical colleges and Deemed Universities, Regulations for Community Health Providers and rating of medical colleges.
- During the last six years, MBBS Seats increased by 87%, from 51,348 seats in 2014 to 96,077 seats in 2022 and the number of PG seats increased by 105% from 2014 (31,185 seats) to 2022 (63,842 seats).
- Further, during the same period, 261 new medical colleges have been established and now the country has 648 (Govt: 355, Pvt: 293) medical colleges.
- Under the Central Sponsored Scheme for establishment of new medical colleges, establishment of 157 medical colleges have been approved in three phases, of which 93 are functional and remaining will be functional in a few years. Of these 157 colleges, 39 are coming up in the Aspirational Districts of the country thereby addressing the issues of inequity in medical education.
- Rationalization of Minimum Standards Requirements (MSR): The MSRs for establishment of medical colleges have been streamlined. This will reduce the cost of establishment of new medical colleges and increase intake capacity.
- Two years post MBBS Diplomas by National Board of Examinations: Keeping in view the importance of Diploma courses to meet the shortfall of postgraduate students and augment healthcare in remote parts of the country, the National Board of Examinations (NBE) has launched diplomas in eight disciplines namely – Anaesthesia, Gynaecology & Obstetrics, Pediatrics, ENT, Ophthalmology, Family Medicine, Tuberculosis & Chest Diseases and Medical Radiodiagnosis.
- District Residency Scheme for Post-Graduation: The MCI has also notified a Scheme known as District Residency Scheme for compulsory three months training of PG medical students at District Hospitals, an essential component of postgraduate medical training curriculum. Under the Scheme, the second/third year PG students of medical colleges would be posted in the district hospitals for a period of three months.
- The constitution of the National Medical Commission has ushered in a landmark reform in the sector of Medical Education. On similar lines, the Government is striving to bring institutional reforms in nursing and dental education sectors by bringing reformative legislations to replace the existing Indian Nursing Council Act, 1947 and Dentists Act, 1948. To address the long standing vacuum of a regulatory body for various professions included in the allied and healthcare sector, a National Commission for Allied and Healthcare Professions Act 2021 has already been enacted. The basic premise and principled change that is happening in all these professional education sectors is that the Regulator is now being ‘selected on merits’, as opposed to an ‘elected’ regulator.
- Indian Council of Medical Research (ICMR)
Indian Council of Medical Research (ICMR), New Delhi, is the apex body in India for the formulation, coordination and promotion of biomedical research and is one of the oldest medical research bodies in the world now under the Department of Health Research (DHR) in the Ministry of Health and Family Welfare, Govt of India.
The Council’s research priorities coincide with the National health priorities such as control and management of communicable diseases, fertility control, maternal and child health, focussing on nutritional disorders, developing alternative strategies for health care delivery, containment within safety limits of environmental and occupational health problems; research on major non-communicable diseases like cancer, cardiovascular diseases, blindness, diabetes and other metabolic and haematological disorders; mental health and drug research (including traditional remedies). All these efforts are undertaken with a view to reduce the total burden of disease and to promote health and well-being of the population.
ICMR has also demonstrated its commitment to the future of medical research through its professional development training and capacity building. This includes training programs, workshops, and short-term research studentships for those preparing for a career in medicine and medical research. It also includes research fellowships and short-term visiting fellowships for upcoming researchers to expand their skills and knowledge early in their career. ICMR also offers Emeritus Scientist positions to enable retired medical scientists and teachers to continue to carry out research on specific topics.
The impact of ICMR spans across the globe with research collaborations spanning every continent. Through ICMR’s Memoranda of Understandings (MoUs), ICMR has partnered with leading universities from around the world to concentrate efforts on leading health issues such as cancer, diabetes, infectious diseases, and vaccine development. These collaborations facilitate the exchange of scientific information, training, joint projects, and co-authorship of meetings, workshops, seminars, and symposia presentations.
8.1 Intramural Research
Intramural research is carried out through a countrywide network of 27 institutes/centres with multiple field stations, 14 ICMR Institutes work in the area of communicable diseases; 6 in Non-Communicable Diseases, 1 in diseases related to Reproductive and Child Health (RCH); 1 in nutrition and nutritional deficiencies, 3 in disease related to Basic Medical Sciences including haemoglobinopathies and traditional medicine, 1 in the area of animal breeding and research and 1 is patient care and research centre.
8.2 Extramural Research
Extramural research of ICMR is promoted through – Setting up Centres for Advanced Research in different research areas around existing expertise and infrastructure in selected departments of Medical Colleges, Universities and other non-ICMR Research Institutes. Task force studies are also carried out which emphasize a time-bound, goal-oriented approach with clearly defined targets, specific time frames, standardized and uniform methodologies, and often a multi-centric structure.
Open-ended research on the basis of applications for grants-in-aid received from scientists in non-ICMR Research Institutes, Medical colleges, Universities etc. located in different parts of the country.
8.3 Achievements during the year:
The major activities and achievements of ICMR are given below:
Outbreak Investigation & Pandemic Preparedness
- Monkey Pox: ICMR conducted the investigation and diagnosis of Monkey Pox Virus in India. It also isolated and cultured the MPV and called for LOI from companies for diagnostic kit and vaccine development. Memoranda of Understanding have been signed with three companies for the validation of the diagnostic kits for monkey pox.
- Lumpy Skin disease in Cattle: Laboratory investigations for Lumpy Skin Disease Virus outbreak were developed to support the regional veterinary diagnostic laboratories.
- Dealing with COVID-19 Pandemic: In the fight against COVID-19, the Indian Council of Medical Research (ICMR), played the crucial role from setting of laboratory network across the country to isolating the virus, doing genetic characterization, genome sequencing, developing newer diagnostics, and research leading to the vaccine development in less than a year time which signifies our science capabilities. A pioneering initiative was undertaken to deliver vaccines and medical supplies through drones in difficult terrains. Recently, ICMR studies have demonstrated that appropriate use of non-rebreather masks during oxygen therapy in covid patients help reduce the risks of black fungus. Also, another study reported that vaccines saved hospitalized patients and prevented deaths.
- Mobile BSL-3 Laboratory: India’s first Biosafety level-3 containment mobile laboratory was launched in Nashik, Maharashtra. The mobile laboratory has been set up to investigate newly emerging and re-emerging viral infections that are highly infectious and of lethal potential to human beings in field settings. This initiative will propel the nation’s scientific and diagnostic capability: a level unseen within Asia and ensure quick containment of Infectious Disease Outbreak.
- Zonal NIVs & BSL-3 & BSL-4 Laboratories: Under Pradhan Mantri Ayushman Bharat Health Infrastructure Mission, the expansion of existing research capacity would be undertaken for infectious disease emergency preparedness. Provisions have been made in the budget to decentralize the load on National Institute of Virology, Pune through establishment of 4 regional national institute of virology in four regions (North, South, East and Central) of the country. Recently, foundation stones of 3 Zonal NIVs have been laid at Bengaluru, Jabalpur and Dibrugarh by Hon’ble President of India. These NIV will help in efficiently countering the threats of viral pandemics/epidemics in the future. To build biosafety and biosecurity enabled infrastructure, budget 2021 proposes to upgrade 5 viral research & diagnostics labs into BSL–III laboratories and setting up of 4 mobile BSL-III labs. These will be used in outbreak situations for rapid processing and testing of specimens related to vector borne, human, animal and environment etc.
Promoting Make-in-India efforts:
- Development of Indigenous diagnostic kits: ICMR developed world’s first rapid, cost-effective kits to diagnose haemophilia, von Willebrand disease. In the conventional setting, it requires trained laboratory staff and equipment to diagnose these diseases, and involves a decent cost too. These facilities are not available in the remote areas of our country. However, in the newly developed rapid kit, 2 micro litre blood plasma sample needs to be dropped on a small strip to test for haemophilia, vWD and results are available within 10 minutes. Also, these kits will bring down the cost from current of Rs. 2,000 – Rs. 9,000 to around Rs 28 only. The kit has been approved by DCGI and technology has been transferred to Bhat Bio-Tech India (P) Ltd (BBI), Bangalore, for large scale manufacturing.
- Large Animal Testing Facility at AMTZ: ICMR-DHR Centre of Excellence Large Animal House Facility has been established to bridge the critical gap for product development and fostering Make-In-India initiative at AMTZ, Vizag, Andhra Pradesh. It will support R & D, prototyping and technological assessment of medical devices.
- ICMR & DHR under Medical Device & Diagnostics Mission Secretariat unveiled ICMR-DHR-Centre of excellence (CoE) at 7 IITs for fostering indigenous Product development in Medtech Sector for an Aatma Nirbhar Bharat.
- ICMR-VCRC developed & transferred Make-in-India technology for Bti-mosquito larvicide to Hindustan Insecticide Ltd.
- ICMR in collaboration with TATA MD developed Omisure, a real –time RTPCR test kit for quick detection of Omicron variant of SARS-CoV2.
- Launched “IBD NutriCare App” for Inflammatory Bowel Disease (IBD) Patients. The app provides real time tracking of dietary details and recording of data on a large scale. It provides a recording of diet variables based on nearly 650 Indian food recipes.
Disease Burden/ Surveillance
- National TB Prevalence Survey: The National TB Prevalence Survey of India 2019-2021, the first of its kind in the country in 55 years, found that there are 312 cases per 100,000 population. The report says that for every notified case of the deadly infectious disease, the actual prevalent number of cases was 2.84. A majority of patients (64%) did not seek treatment, mostly because they ignored the symptoms or did not recognise them. All these findings would help in guiding policies towards TB elimination.
- India Hypertension Research Initiative (IHMI): IHCI is ICMR’s collaborative initiative with MoHFW, WHO & state governments aimed to reduce deaths from heart attacks & strokes. It is playing a critical role in regularizing hypertension treatment across primary care facilities in 100 districts in 23states by providing free drugs & quality treatment to 34lakh patients. This initiative is a progression towards ensuring availability of quality healthcare for Indians The tech driven innovations in the IHCI program allowed India to track over 2 million patients digitally, with minimal burden on human resources and on clinics. 50% patients tracked in real time achieved BP control. The initiative was awarded 2022 UN Inter-Agency Task Force and the WHO special program on Primary Health Care Award during United Nation General Assembly on 21st September 2022.
- National List of Essential Medicines (NLEM): Recently, The National List of Essential Medicine (NLEM) was launched by Hon’ble HFM, Dr Mansukh Mandaviya. The revised NLEM was developed under the chairmanship of Secretary, DHR & DG, ICMR. The primary purpose of NLEM is to promote rational use of medicines considering the three important aspects i.e., cost, safety and efficacy. It also helps in optimum utilization of healthcare resources and budget; drug procurement policies, health insurance; improving prescribing habits; medical education and training for UG/PG; and drafting pharmaceutical policies. In NLEM, the medicines are categorized based on level of healthcare system as- P- Primary; S- Secondary and T- Tertiary.384 drugs have been included in this list with addition of 34 drugs, while 26 from the previous list have been dropped. The medicines have been categorized into 27 therapeutic categories.
- Launched National Hospital based Registry on Venous Thromboembolic Disorders ((i-RegVeD) that aims to establish a nationwide surveillance network through selected hospitals and collect data for generating evidence on VTE prevalence for planning response, and strengthening healthcare facilities across different treatment settings.
- Launched Indian catalogue of Mycobacterium TB mutation & their association with drug resistance interim report.
- ICMR with collaboration of WHO India Released mapping, size estimation and risk behaviour survey among key population groups in virtual space – a basic guide.
Regulatory/Policy/Guidelines
- Launched the “ICMR/ DHR Policy on Biomedical Innovation & Entrepreneurship for Medical Professionals, Scientists and Technologists at Medical, Dental, Para-Medical Institutes/Colleges“: The Policy will promote interdisciplinary collaboration, innovation, technology development, skill development and foster entrepreneurship development & Make-in-India product development for societal benefit. DHR- ICMR formulated this Policy in consultation with other Government Department/ Ministries/Organisations such as DPIIT, DST, WIPO, DSIR, AIIMS, IIT Delhi etc. This Policy is an attempt to enable medical institutions to actively support their personnel to contribute in innovation and entrepreneurship associated activities. This Policy resonates with the motto of Hon’ble Prime Minister to “Innovate, Patent, Produce and Prosper”
- Developed another 2 standard treatment workflows for TB management and 54 other disease conditions respectively.
- Developed standard operating procedure for foodborne pathogen survey & research network, North-East India. It is one step towards reduction of foodborne infections & outbreaks.
- Developed a guidance document for use of drones in healthcare to help state health authorities with the delivery of medical supplies using drones.
- Developed guidelines for the management of type 1 diabetes mellitus (T1DM).
Infrastructure
- NIIH-Centre for Research, Management and Control of Haemolglobinopathies, Chandrapur: This centre will cater to the needs of entire Vidarbha region especially in the area of sickle cell anaemia. There are around 4,00,000 Sickle cell disease patients in this region along with approximately 40,00,000 sickle cell carriers. The Centre has recently been inaugurated by Hon’ble PM on 11th December 2022.
- National Animal Resource facility for Biomedical Research, Hyderabad: The State-of-the-art infrastructural facility is a single stop solution to provide quality services in support of biomedical research and training with adherence to the highest international standards for humane and ethical animal care and use. The 100-acre campus consists of 18 buildings equipped with advanced facilities for animal breeding and research is built at the cost of over 300 Crores. The facility was inaugurated by Hon’ble HFM on 17th December, 2022.
- National Institute of One Health: The government of India has taken an important and crucial step towards the achievement of One Health goal through establishment of a dedicated infrastructure in the form of National Institute of One Health (NIO) at Nagpur. ‘One Health’ approach will help in managing emerging epidemic threats of zoonotic diseases based on human, animal and environment interface. The foundation stone was laid by Hon’ble PM on 11th December 2022.
- Laid the foundation stone of the International Centre of Excellence For Training In Medical Entomology (ICETIME) at ICMR-VCRC, Puducherry. ICETIME will help in meeting the growing demand for trained manpower in the field of public health entomology at regional, national & global levels.
- The mandate of ICMR – National Institute for Research in Reproductive Health, Mumbai (ICMR-NIRRH) has been expanded to include Child Health and thus was renamed as ICMR – National Institute for Research in Reproductive and Child Health (ICMR-NIRRCH).
International Collaborations
- Launched ICMR-NIAID-BMGF Clinical Research Fellowship Programme. This fellowship will advance discovery to improve clinical practices and serve public health causes in both the countries.
- MoU signed between ICMR and NIH for Cooperation on An International Center of Excellence in Research in Chennai, India
- LoI signed between the Indian Council of Medical Research (ICMR), Department of Health Research (DHR) and the Ministry of Health & Social Protection and The Ministry of Science, Technology & Innovation, Colombia
- MoU signed between the ICMR, Department of Health Research (DHR), Ministry of Health & Family Welfare, Government of India and the University of Oxford, United Kingdom in the field of collaborative research on infectious diseases commonly affecting socially marginalized groups
- MoU between the ICMR, Department of Health Research (DHR), Ministry of Health & Family Welfare, Government of India and Deutsche Forschungsgemeinschafte.V. (DFG), Germany. The MoU will focus on Indo-German collaborative research in the areas of Toxicology, Neglected tropical diseases and Rare diseases.
- A Tripartite MoU between ICMR, Department of Biotechnology, Govt. of the Republic of India (DBT) and International AIDS Vaccine Initiative (IAVI), USA. It focusses on to participate and collaborate to prevent, diagnose and/or treat diseases of concern in India and globally, including HIV, Tuberculosis, emerging infectious diseases like COVID-I9 and other global health threats, across Product development including translational research, preclinical and clinical development, community engagement and socio-behavioural research, low cost manufacturing, public health access, etc
8.4 North Eastern Indira Gandhi Regional Institute Of Health And Medical Sciences (NEIGRIHMS), Shillong, Meghalaya
NEIGRIHMS is a Super Specialty teaching Institute established in 1987 in Shillong under the Meghalaya Registration of Societies Act 1983 with an objective to provide advanced and specialized medical facilities of the highest level in selected specialties, and to serve as a Regional Referral Service Centre for comprehensive health care to the people in North Eastern States. It has been designed as a Postgraduate Medical Institute in the lineage of AIIMS, New Delhi and PGIMER, Chandigarh. The Institute is under the administrative control of the Ministry of Health & Family Welfare, Government of India.
The Institute is presently having 30 fully functional Super Speciality and Speciality departments. It is offering super specialty services in Cardiology, CTVS, Neurology, Neurosurgery, Surgical Oncology and Urology, besides specialty services in General surgery, General Medicine, Paediatrics, Obstetrics & Gynaecology, ENT, Orthopaedics, Dentistry, Psychiatry, Radiotherapy, TB & Respiratory Diseases, Dermatology and Ophthalmology. These departments are very well supported by the departments of Radiology, Anaesthesiology, Pathology, Microbiology, Forensic Medicine, Biochemistry, Anatomy, Community Medicine, Pharmacology, Hospital Administration and Physiology. It is well equipped with all basic as well as advanced equipment like CT scan, 1.5 Tesla MRI, Digital Mammography system, Fully automated High Vacuum Double Door Steam Sterilizer Unit and Washer Disinfector, etc.
The hospital presently has 628 beds including 104 ICU beds with ventilators, out of which 280 beds are designated for COVID-19 including 43 ICU beds designated for COVID-19, other ICU beds includes Medical Critical Care Unit with 15 beds, Anaesthesia Critical Care Unit with 16 beds, CTVS ICU with 10 beds, ICCU with 11 beds, Paediatric ICU with 14 beds, Neonatal ICU with 6 beds each.
NEIGIRHMS is also designated as Mentor institute for the entire North Eastern States for COVID Testing facilities by ICMR. Various Covid -19 Testing facilities like RTPCR, TruNAT & CB NAAT are available in the Institute round the clock.
Academic Activities:
The Institute is conducting Post Doctoral (DM), Post-Graduate (MD/MS), Under Graduate (MBBS), M. Sc (Nursing) & B. Sc (Nursing) Courses.
- MBBS Course:
The Under Graduate (MBBS) course was started in the year 2008 an annual intake of 50 MBBS students per year which will be increased to 100 MBBS annual from the next academic session. Infrastructure including college building and hostel buildings are being readied.
Allocation of MBBS seats
The distribution of MBBS seats is as under:
| Sl No. | Name of the State | Number of seats |
| 1 | Meghalaya | 09 |
| 2 | Nagaland | 08 |
| 3 | Arunachal Pradesh | 04 |
| 4 | Mizoram | 03 |
| 5 | Central Pool (15% of total seats) | 08 |
| 6 | Open for all NE states | 18 |
| Total Seats | 50 |
- Post Graduate (MD/MS) courses:
The Institute is running PG (MD/MS) courses in the following departments:
MD Pathology (3 seats), MD Microbiology (3 seats), MD General Medicine (2 seats), MD Radiodiagnosis (2 seats), MD Dermatology (2 seats), MD Obstetrics & Gynaecology (2 seats), MD Anatomy (2 seats), MS Ophthalmology (2 seats), MD Forensic Medicine (3 seats), MS ENT (2 seats). Post Graduate course in Orthopeadics for 2 seats will be started from the next session.
- Post Doctoral (DM) course:
DM Cardiology course was started in 2012 with an annual intake of 2 (two) seats. In addition the Post Doctoral course in DM Neurology with an annual intake of 2 (two) seats has been started in the current academic session.
- Nursing Education:
The B. Sc Nursing course with 50 annual intake was started in the year 2006. The students’ intake will be increased to 100 seats from the next Academic session. Infrastructure including college building and hostel buildings are being readied.
M Sc Nursing Course with 10 students intake per year was started from the year 2016.
Academic Achievements:
- Till date 15 batches of MBBS students have been admitted and 10 batches passed out.
- Number of PG Students admitted is 216 and 133 PGs in various specialties have completed their course.
- Number of DM Cardiology admitted is 20 while 8 batches of DM (Cardiology) have completed the course.
- Number of M. Sc (Nursing) Students admitted is 66 and 4 batches have completed their course.
- Number of B. Sc (Nursing) Students admitted is 850 and 12 batches have completed their course.
- National and International Conferences, symposia, workshops, teleconferences and tele-medicine workshops are held in collaboration with other institutes in the country, involving various other funding agencies for research, especially ICMR, HRD, DST etc. It has been in the forefront with its research publications of appreciable impact on need-based, indigenous projects.
The Radiology Department of the Institute is imparting 6 (six) months training in Ultrasonography to the in-service MBBS doctors nominated by the Government of Meghalaya. The Tomo Riba Institute of Health & Medical Sciences (TRIHMS), Government of Arunachal Pradesh is deputing it Nursing & Technical Staff to undergo training in Cath Lab under the Department of Cardiology. The TRIHMS has also signed an MOU with NEIGRIHMS for training of their DNB candidates in selected departments of the Institute. The Institute is also providing clinical experience to the GNM & B. Sc (Nursing) students of both Government and Private Nursing Institute’s of the State of Meghalaya. The NMC has earmarked 4 (four) seats at NEIGRIHMS to Foreign Medical Graduates (FMGs) to undergo 1 (one) year Compulsory Rotatory Internship Programme.
The Institute has also allocated 8 (eight) Supernumerary Seats under PMSS Scheme for UTs of J&K and Ladakh students and 25% Supernumerary seats for Foreign students at College of Nursing, NEIGRIHMS, Shillong.
Management of the Institute:
The Governing Council of the Institute is the highest authority of the Institute headed by the Union Health Minister as its President with 27 Other Members. The Executive Committee is chaired by the Secretary, Ministry of Health & Family Welfare, Govt. of India. The other committees have also been constituted such as Standing Finance Committee, Standing Selection Committee, and Academic Committee, etc. The Director, NEIGRIHMS is the Chief Executive Officer of the Institute. All administrative and academic activities are under his control.
The Dean (Academics) is the overall incharge of the academic activities of the Institute and the Dean (Research) looks after the research activities. The Medical Superintendent is the overall in-charge of the hospital who looks after the day to day functioning of the hospital. The functioning of the different departments is directly under the respective Heads of Department. Key areas such as the Casualty, CSSD, Stores, Hospital Waste Management, etc are looked after by designated officers under the supervision of the Medical Superintendent.
Sanctioned strength and incumbency position:
Presently the Institute is having a total of 1152 manpower in position including Faculty, Group A, B & C posts against the sanctioned strength of 1823 posts. The Institute has been making efforts to fill up the vacant faculty posts to augment the teaching faculty in different Departments of NEIGRIHMS. The total number of faculty in position is 88 Nos. (Excluding 7 who are yet to join) against the sanctioned strength of 141 posts. The Institute till date is having 58 Nos. of Senior Residents in position out of 105 posts and 76 Junior Residents in position out of 84 posts.
Major high end equipments
The hospital has 16 Operation Theatres including 8 Modular OTs catering to various speciality and superspecialty Surgical disciplines. Specialty Surgical Discipline’s includes General Surgery, ENT, Ophthalmology Obstetrics & Gynaecology, Orthopaedics and Trauma. Superspecialty Surgical Disciplines includes Urology, Neurosurgery, Oncosurgery, Cardio Thoracic and Vascular Surgery.
State of Art Radiology & Imaging Department exist with various radiology equipments including 128 slice CT scan, 1.5 Tesla MRI, Digital Radiography and Digital Radio Fluoroscopy system, various advance Ultrasounography systems, Mobile DSA system, Mammography, DEXA Scan.
The Institute also has the following High end Medical Equipments including:
Cathlab, Lithotripsy Machine, Trans Esophageal Echocardiography, Stereotactic Navigational System for Neuro surgery, CUSA, ECMO, Holmium LASER, Virtual Dissection Table, Virtual Autopsy System.
The hospital has got a Gas Manifold System with centralized pipelines. It has got the following oxygen supply systems:
- 2 Nos. Liquid Medical Oxygen Plant each having 10 KL capacity.
- 2 units of PSA (Pressure Swing Adsorption) plants each having capacity of 300L/min and 700 L/min respectively
- 258 Nos. Oxygen cylinders D-type.
Health Schemes:
NEIGRIHMS has been successfully operating the central schemes like RAN, HMDG, JSY, JSSK, PMNRF and also the Government sponsored Insurance Schemes like Pradhan Mantri Jan Arogya (PMJAY). The Institute entered into an agreement with Government of Arunachal for having cashless treatment for the people of Arunachal at NEIGRIHMS under the Chief Minister Arogya Arunachal Yojana (CMAAY) Scheme (Health Insurance Scheme).
NEIGRIHMS also received the award “Highest Number of Claims” among smaller States for PMJAY.
Hospital Statistics w.e.f 01.01.2022 to date
| Patients availing OPD & IPD services | Operation conducted | Patient availing OPD, IPD services from Meghalaya | Patient from N. E. India excluding Meghalaya | Patient from outside the country | |||||
| OPD | IPD | Major | Minor | OPD | IPD | OPD | IPD | OPD | IPD |
| 284865 | 11318 | 2832 | 1399 | 182706 | 8000 | 99764 | 3269 | 553 | 8 |
- Investigations and Diagnostic Procedures
| Pathology | Microbiology | Bio Chemistry | Radiology |
| 652080 | 319593 | 1106771 | 93094 |
- Procedures
| General Medicine | ENT | Dermatology | Neurology | Urology | Orthopaedics | Gynaecology | Cardiology | CTVS | Blood Bank |
| 12286 | 4136 | 2055 | 2206 | 2888 | 1284 | 5954 | 35505 | 135 | 3873 |
Awards/ Achievements / Activities /Mile Stone Achieved / Individuals :-
| Sl | Department | Achievements / Activities /Mile Stone Achieved / Individuals |
| 1. | Surgical Oncology |
|
| 2. | Radiation Oncology |
|
| 3. | Biochemistry |
|
| 4.
|
Medical Education Unit |
|
| 5. | Radiology |
|
| 9. | Hospital Infection Control |
|
| 10. | Ophthalmology |
|
| 11. | Pharmacology |
|
| 12. | Orthopedics | Prof. BhaskarBorgohain, HoD Orthopedics, NEIGRIHMS developed a new device with IIT Guwahati and Applied for Patent (Ref no. 348843-001, Kolkata Patent office) for new hip fracture fixation (named as DODO device) in collaboration with IIT Guwahati team on 03-09-2021.
Prof Bhaskar Borgohain was awarded Vocational Excellence Award by the Rotary Club at Raj Bhawan,Shillong and also the Dr.Bhupen Hazarika Humanitarian Award 2022 at the JyotiChitrabanCoplex, Guwahati, Assam. |
| 13. | Community Medicine | Establishment of new Urban Health Training Centre, Nongmensong in 2022. |
| 14. | Pathology |
|
| 15. | General Medicine | World Diabetes Day 2022 was celebrated by the Department of General Medicine, NEIGRIHMS, Shillong on the 14th November, 2022. As part of the celebration the following were undertaken:
|
| 16 | Pediatrics | Dr. Himesh Barman, HoD Pediatric, NEIGRIHMS was awarded
Dr. Rosina Ksoo, Assistant Professor, Pediatric was in-Charge of creating the 24 bedded COVID-19 Pediatric ICU at NEIGRIHMS during the Pandemic.
The Neonatal Intensive Care Unit (NICU), Department of Pediatrics was adjudged theHand Hygiene Champions for the year 2022 at the Global Hand Washing Day 2022.
It was also awarded the 3rd Prize in Undergraduate Pediatric Quiz held in Guwahati. |
| 17 | Dentistry | The department won the 1st Prize National Award on the observance of Word Oral Health Day on March 20, 2022. |
| 18 | Obs&Gynae | Dr. Nalini Sharma, Dr. Ananya Das and Dr. Wansalan K Shullai Received Fellowships in Minimum Access Surgery.
Dr. Wansalan K Shullai Won the Best Oral Paper presentation award at the 12th NEIGRIHMS Annual Day Ceremony on the 5th March, 2022. |
| 19 | Forensic Medicine | Virtual Autopsy – The first case was subjected to virtual autopsy in February 2022 in addition to routine conventional autopsy.
Modernization and renovation of mortuary complex with additional rooms for housing CT scan, consoles and workstation, conference room with virtual dissector and restrooms.
Launch of Gas Chromatography/Mass Spectrophotometry (CG/MS) for analysis of common poisons. |
| 21 | Neuro Surgery | Admission to Global Spine Fellowship by Arbeitsgemeinschaft fur osteosynthesefragen (AO) Spine society, Switzerland of our faculty DrBinoy Kumar Singh
PMJAY ward for maximum surgeries in peripheral tertiaty care Institute Clean India -Healthy India, best kept ward, 2nd prize to Neurosurgery ward |
| 22 | Vigilance Section | Prof. P. K. Bhattacharya, Professor & Head & Vigilance In-Charge, organized Vigilance Awareness Week from 30-10-2022 to 06-11-2022 in NEIGRIHMS. |
NEIGRIHMS was awarded the 1st Prize in “KAYA KALP” with a prize amount of Rs. 1.5 Crore under the Central Government Hospitals category of the KAYA KALP awards, for maintaining high standard for sanitation and hygiene in the country.
Grant-in-aid and Budget
Besides receiving grants-in-aid from the Ministry the Institute receives grants for implementation of various projects such as GFATM, Cancer Atlas and other research projects from ICMR, DBT etc. and grants from schemes like RANS, JSY etc.
| Budget Estimate for
2022-23 |
Allocation for 2022-23 | Funds Released by MoHFW as on November 2022 |
| 486.19 Crs | 456.00 Crs | 332.78 Crs. |
MAJOR EXPANSION PROJECTS OF NEIGRIHMS
- Expansion of Nursing College with Hostel (from 50 to 100 intake).
- Establishment of Under Graduate Medical College with Hostel for 100 intake with hostels for 600 students.
- Setting up of Regional Cancer Centre with 252 bed capacity with Patient Guest House of 28 rooms.
On certification of completion of the following buildings by Project consultant M/s HSCC and after obtaining the occupancy certificate and Fire NOC from MUDA and Fire & Emergency services –Government of Meghalaya respectively followed with joint inspection between NEIGRIHMS, M/s HSCC and M/s L&T, these buildings have been taken over :-
| Sl No | Name of Building | Date of taking Over |
| 1. | Nursing Hostel -1 | 05.09.2022 |
| 2. | Nursing Hostel -2 | 05.09.2022 |
| 3. | New Guest House | 10.10.2022 |
| 4. | Radiotherapy Floor ( 1st Floor –RCC) | 11.10.2022 |
| 5. | Under Graduate Hostel -1 | 26.10.2022 |
| 6. | Under Graduate Hostel -2 | 26.10.2022 |
| 7. | Internee Hostel | 26.10.2022 |
| 8. | Nursing College & Dinning | 26.10.2022 |
| 9. | Under Graduate Medical College | 07.11.2022 |
| 10. | Under Graduate Hostel -3 | 16.11.2022 |
| 11. | Under Graduate Hostel – 4 | 16.11.2022 |
Status of the Construction as on 7.12.2022 is as follows:-
Out of 12 (Twelve) buildings, 10 (ten) buildings and the Radiotherapy Floor at the RCC has been taken over by Institute, as indicated above.
RCC is about 98 % completed with external development works remaining and expected to be completed by December 2022 (end)
| Total Project Cost
( Excluding Escalation ) |
Rs 363.99 Cr |
| Total Running Bill paid to M/s HSCC – M/s L&T | 334.50 Cr
Financial achievement – 92% |
| Total Consultancy paid to M/ s HSCC | 19.04 Cr
( Inclusive of GST) |
Capacity of Buildings etc:-
| FACILITY | CAPACITY |
| Nursing college | 100 Students / Year |
| Nursing hostel – 1 | 68 Rooms, Capacity 136 students |
| Nursing hostel – 2 | 87 Rooms, Capacity 174 students |
| Nursing dining | Capacity – 390 Seats |
| Guest house | 28 Rooms, Capacity 52 persons |
| UG Hostel – 1 | 76 Rooms, Capacity 92 students |
| UG Hostel – 2 | 91 Rooms, Capacity 109 students |
| UG Hostel – 3 | 91 Rooms, Capacity 109 students |
| UG Hostel – 4 | 74 Rooms, Capacity 88 students |
| Internee hostel | 84 Rooms, Capacity 100 students |
Regional Cancer Centre with 252 bed capacity with Patient Guest House of 28 rooms will have all state of art cancer diagnosis and treatment facilities including Linear Accelerators, Brachy Therapy, CT Simulator, PET CT-Scan, Gamma Camera and various advanced cancer treatment facilities with 6 Modular Operation Theatres. It is also have all state of the are diagnostic radiological equipments including 3 Tesla MRI, 128 slice CT Scan, Digital Mammography, DSA, DRF, DR systems and PACS (Picture Archiving and Communication System). There will be Oncology, Onco Surgery, Radiation Therapy and Onco Imaging Facilities.
8.5 Regional Institute of Medical Sciences, Imphal
Regional Institute of Medical Sciences was set up in 1972 and has been functioning under the Ministry of Health and Family Welfare since 1st April, 2007. RIMS is an Institute of regional importance catering to the needs of the North Eastern Region in the field of medical education by providing undergraduate and post-graduate courses. RIMS, Hospital is a 1,200 bedded teaching Hospital equipped with modern state of the art equipment and teaching facilities. The Hospital provides services to a large number of patients both out-door as well as indoor patients and admit over forty thousand patients in a year. The institute has so far produced 3673 medical graduates and 2225 specialists.
| Sl. No. | Name of Course | Number of seats | Quotas |
| 1 | MBBS | 125 seats per annum | 15% All India Quota |
| 2 | MD/MS/DCP | 156 seats per annum | 50% All India Quota |
| 3 | M. Ch./D.M. | 05 seats per annum | 100% All India Quota |
| 4 | M. Phil. | 06 seats per annum | *Open Beneficiary states of RIMS |
| 5 | B. Sc. Nursing | 50 seats per annum | All Beneficiary states of RIMS |
| 6 | BDS | 50 seats per annum | 15% All India Quota |
| 7 | BASLP | 10 seats per annum | All Beneficiary states of RIMS |
| 8 | M.Sc. (Nursing) | 10 seats per annum | All Beneficiary states of RIMS
& 1 seat earmarked for children of RIMS employee |
| 9 | B.Sc (MLT) | 15 seats per annum | *Open Beneficiary states of RIMS |
| 10 | B.Sc. (MRIT) | 5 seats per annum | *Open Beneficiary states of RIMS |
- The courses being run along with intake capacity in the institute are as follows:
2.1 Allocation of Seats for undergraduate courses:
The number of annual admission to MBBS course is 125 students. The detail of these seats is as under:-
| Sl. No. | Name of State | MBBS | BDS | B.Sc. Nursing |
| 1 | All India Quota | 19 | 7 | – |
| 2 | Arunachal Pradesh | 7 | 4 | 5 |
| 3 | Meghalaya | 13 | 7 | 5 |
| 4 | Mizoram | 7 | 4 | 5 |
| 5 | Manipur | 30 | 13 | 20* |
| 6 | Sikkim | 5 | 3 | 5 |
| 7 | Tripura | 13 | 7 | 5 |
| 8 | Nagaland | 10 | 5 | 5 |
| 9. | NE Open- All Beneficiary states of RIMS (except Assam) | 10 | – | – |
| 10. | EWS | 11 | – | – |
| Grand Total | 125 | 50 | 50 | |
* including 4 seats earmarked for children of RIMS employees.
2.2 Distribution of P.G. seats
50% (73-74) seat distribution of Beneficiary States of RIMS, Imphal
| Course | State | No. of seats | Total seats | |
| Sponsored | Open | |||
| Postgraduate (MD/MS/DCP) | Arunachal Pradesh | 8 | 2 | 10 |
| Manipur | 8 | 2 | 10 | |
| Meghalaya | 8 | 2 | 10 | |
| Mizoram | 7 | 2 | 9 | |
| Nagaland | 7 | 2 | 9 | |
| Sikkim | 7 | 2 | 9 | |
| Tripura | 8 | 2 | 10 | |
| RIMS AIQ Graduate | 2 | 2 | ||
| NON RIMS Graduates of beneficiary States (except Assam) | 5 | 5 | ||
| NE open (Graduates of beneficiary states of RIMS except Assam) | 4 | 4 | ||
| *This Category was made available only for 2022 session due to increased of PG seats. | ||||
| 78 | ||||
2.3 ACADEMIC ACHIEVEMENT
The objective of this premier institute is to impart quality medical education and has produced a number of medical doctors/specialists and health care providers. On the basis of the record maintained by the institute number of the students passed out so far as on 31.10.2022 is as under:
- Total no. of MBBS doctors passed out – 3673
- Total no. of MD/MS/DCP passed out – 2225
- Total no, of M.Ch./students passed out – 28
- Total no. of M.Phil. (Clinical psychology) – 73
- Total no. of B.Sc. (Nursing) Passed out – 329
- Total no. of B.D.S. passed out – 210
- B.Sc. (MLT) passed out – 5
- MANAGEMENT OF THE INSTITUTE
The Institute and its teaching hospital is under the administrative control of the Director, RIMS, Imphal. The Board of Governors of the Institute is headed by the Union of Health and Family Welfare Minister as its President.
The Executive Council is chaired by the Secretary, Ministry of Health & Family Welfare, Govt. of India. The other committees have also been constituted such as Standing Finance Committee, Academic Sub-Committee etc.
The Medical Superintendent is the overall in-charge of the hospital, who looks after the day to day functioning of the hospital. The functioning of the different departments is directly under the respective heads of department. Key areas such as the Casualty, CSSD, Stores, Hospital Waste Management, etc are looked after by designated officers (medical doctors) under the supervision of the Medical Superintendent.
- STAFF STRENGTH IN RIMS
| Sanctioned Posts | Filled |
| 1936 | 1401 |
- NEWLY PROCURED EQUIPMENTS/INSTRUMENTS
The list of newly procured major equipments for RIMS Imphal for the year 2021-2022 are as follows:-
- Five new Modular OTs with all state-of-the art accessories viz. CCTV, locker, OT table, OT light, Modular laminar air flow, medical gas pipeline, high end monitoring have been installed and commissioned in the OT Complex. These OTs have five years warranty from date of commissioning.
- One 64 Slice CT Scan machine has been installed for use in radio diagnosis.
- One High End Surgical Microscope was commissioned in Neurosurgery Department.
- One more 10 KL LMO Cryogenic Plant at RIMS Hospital, Imphal was commissioned for oxygen supply to the critical patients in addition to the one commissioned the previous year.
- One PSA Plant 200 D-Type Capacity for producing oxygen was constructed under PM CARES Fund and 850D- Type Cylinders for storing oxygen and fifty 10 Litre capacity Oxygen concentrate or RIMS Hospital, Imphal were procured and commissioned at RIMS Hospital, Imphal.
- One Surgical Operating Microscope was procured and used in the Ophthalmology Department.
- One 32 Slice CT Scan was installed at Trauma Care Centre.
- Treadmill Test (TMT) and HOLTER System were installed at Department of Cardiology.
- Real Time Virtual Human Physiology Simulator for teaching students in simulated environment was procured and installed in Physiology Department.
- Automated Blood Bank Immunohaemotology was set up in IHBT Department.
- Seven Dialysis machines to cater to the increasing need of dialysis and one CRRT machine were commissioned in the Department of Nephrology.
- OTHER ACHIEVEMENTS
- The Dental College, RIMS, Imphal (which was started in the year 2012) has been functioning from a temporary building. Now, the construction of the new Dental College block comprising of Administration Block, Pre-Clinical Block, Clinical Block and Academic Block was completed and inaugurated on13th June, 2022.
- Newly constructed Paediatric Emergency Block, Department of Paediatrics, RIMS, Imphal was inaugurated on 3rd November, 2022.
- Newly constructed Sushruta Hall (Adjust to Jubilee Hall), RIMS, Imphal was inaugurated on 13th May, 2022.
- Biomedical Waste Incinerator was inaugurated on 20th August, 2021.
- New Electrical Section Building was inaugurated on 2nd February, 2022.
- A new Ladies House – Sanarei House was completed and inaugurated in February 2022
- A new lift for OPD and lecture theatre complex was inaugurated in February 2022.
- New Double storied Toilet Complex for patient parties which includes facilities for transgender/unisex in the OT Complex was inaugurated on 19th February.
- BUDGET
(Rs. in crore)
| Sl. No. | Financial Year | Allocation BE | Release
2021-2022 |
| 1 | 2020-21 | 438.77 | 457.83 |
8.6 Regional Institute of Paramedical And Nursing Sciences (RIPANS), Aizawl, Mizoram
Regional Institute of Paramedical and Nursing Science (RIPANS), Aizawl was set up by the Ministry of Home Affairs, Government of India in 1995-96 to provide Nursing, Pharmacy and Paramedical education to the people of North East including Sikkim and to maintain the pace of Nursing education and Nursing services with other developments in medical and technological services. The institute was transferred to Ministry of Health and Family Welfare w.e.f. 01.04.2007.
At present, the institute is conducting the following Courses:
| Sl. No. | Name of Course | Duration |
| 1. | B.Sc.Nursing | 4 years |
| 2. | B.Sc. MLT (Medical Laboratory Technology) | 4 years |
| 3. | B. Pharm | 4 years |
| 4. | B.Sc.RIT (Radio Imaging Technology) | 4 years |
| 5. | B. Optometry | 4 years |
| 6. | M.Pharm | 2 years |
| 7. | M.Sc.MLT | 2 years |
Achievements (as of 31st November, 2022):
-
- Total strength of students in various Courses, students newly admitted and number of passed out students is shown as under:
| Sl.
No. |
Name of Course | Total strength of students | No. of students newly admitted in 2021-22 | No. of students passed out in 2021 |
| 1. | B.Sc. Nursing | 171 | 52 | 33 |
| 2. | B.Sc. MLT (Medical Laboratory Technology) | 147 | 43 | 32 |
| 3. | B. Pharm | 155 | 41 | 26 |
| 4. | B.Sc. RIT (Radio Imaging Technology) | 134 | 37 | 29 |
| 5. | B.(Optometry) | 128 | 37 | 34 |
| 6. | M. Pharm | 40 | 20 | 15 |
| 7. | M.Sc.MLT | 16 | 8 | |
| 8. | M.Sc Nursing | 15 | 15 | |
| TOTAL | 806 | 253 | 169 | |
-
- About 99% of passed out students are getting placement in various Central/State Government Institutes/Departments and private establishments such as CSIR Laboratories, AIIMS, Safdurjang Hospital, NEIGRIHMS, RIMS, NIPER, GNRC, AMRI Hospital, Apollo Hospital, Birla Heart Institute, Fortis Hospital, TATA Hospital, NIT, Mizoram University, Assam Downtown University, Assam Technical University, NATCO Pharma Ltd., Torrent Pharmaceuticals Ltd., CIPLA, etc. and abroad such as Australia, USA, Canada, Ireland, England, Norway, Singapore etc.
In addition, many students qualified for the All-India GPAT examination conducted by National Testing Agency (NTA).
-
- As per instruction of Ministry, recruitment process for filling up of various vacant posts at RIPANS is being initiated and action plan is being updated from time to time.
- Project of Development of RIPANS:
- Approval for the Project of Development of RIPANS at an estimated cost of Rs. 480.12 crore was conveyed by the Ministry on 27.02.2019.
- Approval to award the work to the lowest bidder at Rs. 217.97 crore was conveyed by the Ministry on 04.01.2021.
- Civil construction work was started on 01.03.2021 and the scheduled date of completion is 28.08.2023
- The main components of the project are as under:
- Construction of-
| Sl.No. | Name of buildings | Main components |
| 1. | Hospital | 100 bedded |
| 2. | Medical Superintendent’s Quarter | Single Unit |
| 3. | Resident Doctor’s Quarter | 22 units |
| 4. | Staff/Nurse Quarters | 18 units |
| 5. | Academic Block-IV | 18 classrooms & 32 labs |
| 6. | Guest House | 10 units |
| 7. | General Hostel Block | 168 single rooms |
| 8. | Indoor Sports Complex and Auditorium |
|
- Opening of 7 new Courses
- Creation of 154 posts.
-
- Financial Position during the year 2021-22:
| (Rs. In Crore) | |||||||
| Sl.
No. |
Particulars | B.E.
(In crore) |
Unspent Balance of the previous year | Amount released by the Ministry | Internal Resources Generated | Expenditure as on 31.03.2022 | Unspent balance as on 31.03.2022 |
| 1 | GIA General | 16.50 | 7.78 | 6.50 | – | 12.50 | 1.78 |
| 2 | Grants for Creation of Capital Assets | 58.87 | 0.09 | 58.72 | – | 58.60 | 0.21 |
| 3 | GIA Salaries | 13.50 | 5.12 | 8.00 | 0.55 | 13.11 | 0.56 |
| TOTAL | 88.87 | 12.99 | 73.22 | 0.55 | 84.21 | 2.55 | |
Note: Unspent Balance of the previous year was remitted to consolidated fund of India.
-
- Financial Position during the year 2022-2023 (upto 30.11.2022):
(Rs. in Crore)
| Sl. No. | Particulars | B.E. (In crore) | Unspent Balance of the previous year | Amount released by the Ministry | Internal Resources Generated | Expenditure as on 30.11.2022 | Unspent balance as on 30.11.2022 | |
| 1 | GIA General | 15.00 | 1.77 | 11.25 | – | 12.20 | 0.83 | |
| 2 | Grants for Creation of Capital Assets | 102.50 | 0.22 | 53.00 | – | 51.73 | 1.48 | |
| 3 | GIA Salaries | 14.50 | 0.56 | 11.25 | 0.38 | 9.86 |
2.33 |
|
| TOTAL | 132.00 | 2.55 | 75.50 | 0.38 | 73.79 | 4.64 | ||
- National Leprosy Elimination Programme (NLEP):
National Leprosy Eradication Programme (NLEP) is a Centrally Sponsored Scheme under the umbrella of National Health Mission (NHM). India has achieved the elimination of leprosy as a public health problem i.e., defined as less than 1 case per 10,000 populations, at the National level.
The NLEP aims at eliminating leprosy in each of the districts by 2030. Under the National Leprosy Eradication Programme action is taken for early case detection; complete treatment of detected cases, and to contain the onset of disease in close contacts of the index cases (persons diagnosed with leprosy).
Major Achievements so far:
- The prevalence rate of leprosy has come down from 0.69 per 10,000 population in 2014-15 to 0.45 per 10,000 population in 2021-22.
- Percentage of Grade-II disability among new cases has come down from 4.61% in 2014-15 to 2.47% in 2021-22.
- Grade- II disability rate per million population has come down from 4.46 in 2014-15 to 1.36 per million population in 2021-22.
- Percentage of child leprosy cases among total new cases has come down from 9.04% in 2014-15 to 5.45% in 2021-22.
- Annual new case detetection rate per 100,000 population (ANCDR/100,000) has come down from 9.73 in 2014-15 to 5.52/ 100,000 in 2021-22.
- Leprosy Case Detection Campign (LCDC) was started in 2016-17 as major initiative to search hidden cases in community. Under LCDC 25.59%, 25.93%, 19.41%, 20.16%, and 25.46% of total new cases were detected in the year 2016, 2017, 2018, 2019, and 2022 respectively.
- National Center for Vector Borne Diseases Control (NCVBDC)
-
- Malaria
- World Malaria Reports for 3 consecutive years have hailed India’s progress in achieving malaria elimination by 2030. As per recently launched, World
Malaria Report (WMR) 2021, India is the only high endemic country which has reported a decline of 25.24% in malaria cases and 4.67% in malaria
deaths in 2020 as compared to 2019. Malaria cases reported in 2021 across the country were 161753 in comparison to 186532 cases in 2020, indicating a decline of 13.28% over the year 2021. In 2022 (till October 2022-Provisionally), there is 15.97% increase in malaria cases, 5.79% increase in falciparum malaria and. 37.50% decrease in malaria deaths compared to corresponding period of 2021. - In 2021, National API was 0.12 per 1000 population and total 34 States/UTs have achieved API less than one except Tripura (2.58) and Mizoram (6.51).
Only 24 districts have Annual Parasite Incidence (API) one and above.
In 2021, a total 125 districts in the country have reported ‘zero malaria cases’.
At present, 33 States and UTs have made Malaria a notifiable disease and
remaining States & UTs (Bihar, Meghalaya, and Andaman & Nicobar Islands)
are under process to make malaria a notifiable disease. - On World Malaria Day 2022, 18 states /UTs (Andaman and Nicobar Islands, Andhra Pradesh, Arunachal Pradesh, Assam, Bihar, Chhattisgarh, Daman Diu Dadra & Nagar Haveli, Gujarat, Jharkhand, Karnataka, Madhya Pradesh, Meghalaya, Nagaland, Odisha, Tamil Nadu, Telangana, Uttar Pradesh, and West Bengal were awarded for significant progress towards malaria elimination.
- Malaria program review (MPR) of nine states was conducted in April 2022 by independent multi-disciplinary technical expert teams. Based on the MPR recommendations, formulation of National Strategic Plan (NSP) for Malaria elimination 2023-2027 is under process.
- In 2020-21, 2.52 crore LLINs were centrally supplied to 11 states (Andhra
Pradesh, Bihar, Chhattisgarh, Gujarat, Jharkhand, Karnataka, Maharashtra, Odisha, Telangana, West Bengal and Dadar Nagar Haveli and Daman & Diu) in the high endemic areas and 11.45 lakh LLINs were supplied to the Chhattisgarh state in 2021-22. - In 2022, National level refresher trainings (5 batches) and External
Competency Assessment (ECA) (1 batch) on malaria microscopy was
conducted at NCVBDC for certification of Lab technicians from different
Regional office of Health & Family welfare (ROHFW), States and UTs.
Malaria microscopy has also been strengthened by National Refresher
training and certification of a core group of Laboratory Technicians from
different States. There are 11 Level-1 and 18 Level-2 WHO certified
Laboratory technicians for strengthening microscopic activity and lab capacity building. - Implementation of Integrated Health Information Platform (IHIP) – malaria in Odisha and Himachal Pradesh.
10.2 Kala-Azar
- 776 cases have been reported during 2022 upto October in comparison to 1166 cases reported during corresponding period of 2021, reporting a reduction of 33.44% of cases.
- Till October 2022, 99.84% Kala-azar endemic blocks have achieved the elimination target of <1 KA case per 10,000 population at block level. Only one block of Jharkhand is yet to achieve the elimination target.
- Based on the KA Independent Assessment and Kala-azar situational assessment findings, implementations of KA activities have been strengthened. High priority villages have been identified for an intensified action plan. SOPs for active case detection, outbreak management, Operational Definitions in Kala-azar Elimination Programme and guidelines for certification and awards for achieving kala-azar elimination status under the national kala-azar elimination programme have been prepared and duly disseminated to the states for necessary action.
10.3 Dengue & Chikungunya
- The number of identified Sentinel Surveillance Hospitals (SSHs) has been
increased from 713 in 2021 to 783 in 2022 (till 30th November, 2022).
Case Fatality Rate (CFR) for Dengue (deaths per 100 cases) has maintained at <1% in 2022 (till 30th November). - Hon’ble HFM virtually reviewed VBDs situation and preparedness with the State Ministers of 13 States/UTs on 8th July, 2022. For capacity building of entomologist three trainings were conducted on 7th – 11th March & 28th March to 1st April and 12th-16th September, 2022.
- Updated guidelines on Clinical Management of Dengue and Chikungunya and conducted National Training of Trainers on Clinical Management of Dengue and Chikungunya on 28th – 29th November, 2022 at Delhi on these guidelines.
- Operational Guidelines for prevention and control of Aedes mosquitoes in Hospital settings’ shared with Central Govt Hospitals in Delhi and uploaded on website. ‘Manual on Integrated Vector Management in India 2022’- Released by Hon’ble HFM on 25th April, 2022 and uploaded on website.
- First RRM for Dengue, Chikungunya and Zika situation and preparedness of 13 States/UTs (Tamil Nadu, Kerala, Chhattisgarh, Andhra Pradesh, Odisha, Lakshadweep, Karnataka, Maharashtra, Goa, A&N Islands, Madhya Pradesh, Telangana and Puducherry) held at Chennai on 26th- 27th May, 2022.
- Second regional review meeting for Dengue, Chikungunya and Zika situation and preparedness of 13 States/UTs (Gujarat, Rajasthan, Punjab, Haryana, Uttar Pradesh, Uttarakhand, Jammu & Kashmir, Delhi, Bihar, Jharkhand, Chandigarh and DD & DNH held at Ahmedabad on 25th-27thAugust,2022.
- 15 Central teams were deputed to various States/UTs (Andaman & Nicobar Islands, Andhra Pradesh, Dadra & Nagar Haveli, Bihar, Chhattisgarh, Haryana, Himachal Pradesh, Jammu & Kashmir, Karnataka, Kerala, Rajasthan, Tamil Nadu, Telangana, Uttarakhand and Uttar Pradesh) reporting higher number of Dengue cases.
10.4 Japanese Encephalitis
- Out of 60 Pediatrics Intensive Care Units (PICU), 44 PICUs have been made functional (Assam-6. Bihar-7, Tamil Nadu-5, Uttar Pradesh -16 in 15 Dist. and West Bengal-10).
Funds have been provided for all 10 Physical medicine & Rehabilitation
(PMR) Deptts. 8 PMRs are functional (Assam-2, Tamil Nadu-1, Uttar
Pradesh-3 and West Bengal-2). - JE vaccination Campaign mode in children (1-15 yrs.) have been completed in 297 endemic districts. 39 more districts have been identified to cover under the JE vaccination campaign in children.
- 31 districts (Assam (9), Uttar Pradesh (7) and West Bengal (15) have been
covered under Adult JE Vaccination. - 151 Sentinel sites and 15 Apex Referral Laboratories have been identified for diagnosis of JE. 477 JE IgM kits have been supplied in 2022(till 23.11.2022).
10.5 Lymphatic Filariasis
- Out of 328 LF endemic districts, 134 districts have cleared Transmission Assessment Survey (TAS)-1 and have consequently stopped Mass Drug Administration (MDA). Out of 134, TAS-2 is cleared by 123 districts and TAS-3 is cleared by 95 districts till October 2022. During 2022 till October2022 TAS-1, TAS-2 and TAS-3 have been cleared by 2, 3 and 18 districts respectively.
- Out of 328 endemic districts, 133 districts are targeted for MDA during 2022, out of which 90 conducted MDA till October 2022 (78 DA 12 Triple Drug Therapy IDA(Ivermectin+DEC+Albendazole)]. Out of MDA targeted districts 43 districts still need to be covered in 2022. Remaining 61 Districts are either under TAS or pre-TAS.
llumination of New Delhi Railway station and other important buildings in Delhi and other states on 30th January 2022 on NTD day with the objective of creating awareness about VBDs. - Virtual Meeting held with the state and district leadership of four states Bihar, Jharkhand, UP and Odisha under the Chairmanship of Dr.V.K.Paul, member NITI Aayog on 7th February, 2022 with focus on 71 aspirational districts that are endemic for LF.
- Expert meeting regarding DEC fortified salt for Lymphatic Filariasis Elimination was held on 11th April, 2022 under DGHS.
- A virtual meeting was held under the Chairmanship of Health Secretary (H&FW) on 2nd June 2022 to discuss various programmes including VBD’s with State/UTs Health Secretary/Mission Director (NHM).
- Two batches of National Training of Trainers on Morbidity Management and Disability Prevention (MMDP) was organised at Government Medical College Alappuzha, Kerala which is WHO collaborating Centre for MMDP.
- Meeting of the National Programme Managers for Lymphatic Filariasis (LF), Soil-transmitted Helminthiasis (STH) and Schistosomiasis and the Regional Programme Review Group (RPRG) of the South-East Asia Region, New Delhi, 28 June to 1st July 2022.
- Virtual Review meeting held under the Chairmanship of Hon’ble HFM on 8th July 2022 to review the preparedness of Vector Borne Disease (VBDs) including Lymphatic Filariasis (LF) in 13 States.
- Review meeting on Introduction of DEC Fortified Salt in Lymphatic Filariasis Programme held under the chairmanship of DGHS on 12th July 2022.
- National Lymphatic Filariasis Review Meeting under Chairpersonship of DGHS held on 26th and 27th July at New Delhi.
- Conclave on Effective Communication for MDA Mobilization on 28th July 2022 at New Delhi.
- Meeting to review LF situation under Hon’ble HFM held on 12th September 2022.
National TOT on IDA Impact Survey and Brugia TAS with NBS was conducted on 3rd to 4th November2022. - JMM of ELF was conducted from 14th to 25th November, 2022 in 5 states namely Assam, Bihar, Jharkhand, Telangana and Uttar Pradesh.
11 Food Safety and Standards Authority of India (FSSAI):
- Food Safety and Standards (FSS) Act, 2006 was enacted with the objective to consolidate the laws relating to food and for laying down science based standards for articles of food as well as to regulate their manufacture, storage, distribution, sale and import to ensure availability of safe and wholesome food for human consumption . The Food Safety and Standards Authority of India (FSSAI) was established in September, 2008 under the provisions of the FSS Act as the apex authority on all matters of food safety and to ensure safe and wholesome food to consumers.
- FSSAI has constituted 21 subject specific Scientific Panels under Section 13 of the FSS Act, which consist of independent scientific experts, to act as the risk assessment bodies and provide their considered scientific opinion. There is also a Scientific Committee under Section 14 of the FSS Act which is primarily responsible for providing scientific opinion to the Food Authority, general co-ordination necessary to ensure consistency of the scientific opinion, and in particular with regard to the adoption of working procedures and harmonisation of working methods, of the Scientific Panels, opinion on multi-sectoral issues falling within the competence of more than one Scientific Panel and setting up working groups on issues which do not fall within the competence of any of the Scientific Panels. The Scientific Committee and the Scientific Panels meet as often as required to give scientific opinions and recommend on development of food standards.
- During 2022, FSSAI continued to work towards development/revision of science based and internationally benchmarked standards of food products. During 2022, FSSAI notified 14 final notification and 8 draft notifications. The final notifications include standards/revised standards for various articles of food; non-transparent packaging material for water; Ayurveda Aahara; Non-Alcoholic counterpart of alcoholic beverages and falvoured beer etc. Vegan Foods; Advertising and claims regarding virgin coconut oil, chia oil, avocado oil, sunflower seed oil-high oleic acid, and safflower seed oil high oleic acid; change in the nomenclature of Multisource edible vegetable oil as Multi source edible oil; amendment in font size & exemption etc. fermented soybean curd; change in procedure for grant of prior approval; labelling and display amendment regulations related to pan masala and various types of breads. Draft notifications include, inclusion of Pan masala and arecanut based mouth fresheners in food category; allow the declaration of nutritional information such as calorie and carbohydrate content on alcoholic beverages and regarding the revision in the definition of single malt or single grain whisky; use of recycled plastic in food packaging; financial regulations; health supplements, nutraceuticals, food for special dietary use, food for special medical purpose, and prebiotic and probiotic food; transaction of business at its meetings; labelling & display related to front of pack nutritional labelling and high fat, sugar salt etc.
- FSSAI has operationalized an online portal viz. ePAAS (electronic product & claim approval application system) w.e.f. 1st April, 2022. The ePAAS portal facilitates the Food Business Operators (FBOs) to submit requisite fee and application under the FSS (Approval for Non-Specified Food and Food Ingredients) Regulations, 2017. New enhanced portal of Food Safety Training and Certification (FoSTaC 2) launched with features like digital signature on the certificate, attendance and assessment documents upload and training videos upload. The entire training programme is managed by this portal. Integration between Food Safety Compliance System (FoSCoS) and Indian Food Laboratory Network (INFoLNET) is done during the year for seamless flow of lifting of samples and testing of samples through INFoLNET.
- FSSAI notified two new FSS Regulations, viz. FSS (Ayurveda Aahaar), Regulations, 2022 and FSS (Vegan Foods) Regulations, 2022. The objective of the regulations for Ayurveda Aahar is to define Ayurveda Aahar in accordance with the recipes or ingredients and/or processes as per methods described in the authoritative books of Ayurveda listed under ‘Schedule A’ of the regulations. FSSAI formulated a provision for Vegan foods and a logo for easy identification and distinguish from non-vegan foods.
- The “Network for Scientific Cooperation for Food Safety and Applied Nutrition (NetSCoFAN) was established under Section 16(3)(e) of FSS Act, 2006 to have a network of research and academic institutions working in the area of food and nutrition. The NetSCoFAN comprises of the 9 groups in which 11 Lead Research Institutions are working together with other partner institutions, in different areas of food safety and concerns. FSSAI is providing the Annual Financial Grant (currently 20 lakhs per institution) to these institutions.
- Issued guidance notes on fixation of maximum residues limits of pesticides in food commodities; elimination of trans fatty acids; and display of information in food service establishments (Menu Labelling) and published them on the FSSAI’s website.
- All Food Business Operators (FBOs) in the country are required to be registered or licensed under Section 31 of the FSS Act, 2006 to commence or carry on any food business. The process for applying and issuance of licenses and registration of FBOs is completely online through Food Safety Compliance System (FoSCoS). As on 31.10.2022, 55,813 Central Licenses, 9,18,238 State licenses and 41,42,771 Registrations are active. Adding Licenses and Registration granted at Railways Stations and Airports/Seaports, the cumulative figures for the active license and registration becomes 51.2 lakhs. Continuous steps are being taken to simplify the procedure of licensing and registration of food businesses and digitation of enforcement activities.
- The State/UT Governments are primarily responsible for the enforcement of the FSS Act, 2006 in their respective jurisdictions through the institution of the Commissioner of Food Safety. The team under the Commissioner of Food Safety includes Designated Officers (DOs) and Food Safety Officers (FSOs). Food Safety Commissioners (FSCs) in various States and UTs undertake the task of licensing/registration and enforcement through DOs and FSOs. Adjudicating machinery includes Adjudicating Officers (AOs) and Appellate Tribunals, besides Special Courts and Ordinary Civil Court has been set up in States/UTs. Regular follow-ups are being done through quarterly Central Advisory Committee (CAC) meetings. Commissioners of Food Safety of States/UTs have undertaken several special enforcement drives/surveillance campaigns on the directions of FSSAI.
- FSSAI is extending both technical and financial support to the States/UTs for strengthening the Food Safety Ecosystem in the Country through MoU. During 2020-21, Rs. 64.66 Cr to 24 States/UTs and during 2021-22, Rs. 95.49 Cr to 34 States/UTs were released. During the current financial year, Rs. 253.91 Cr have been released till October, 2022 to 34 States/UTs.
- FSSAI has developed the State Food Safety Index to measure the performance of States on various parameters of Food Safety. Index is based on performance of States/UTs on five signigicant parameters, namely Human Resource and Institutional Data (weightage -20%), Compliance (30%), Food Testing Infrastructure and Surveillance (20%), Training and Capacity Building (10%) and Consumer empowerment (20%). The fourth state food safety index for the year 2021-22 was released on 07.06.2022. Among the larger States, Tamil Nadu was the top ranking State, followed by Gujarat and Maharashtra. Among the smaller States, Goa came first followed by Manipur and Meghalaya. Among UTs, J&K, Delhi and Chandigarh secured top ranks.
- During 2022, 11 food laboratories have been recognised/notified and 08 food testing laboratories de-notified under Section 43(1) of FSS Act, 2006 by FSSAI. This has raised the total number of notified food laboratories for primary food sample testing from 222 to 225 till date. All FSSAI notified labs are NABL accredited. In addition, 2 food laboratories have been recognised/notified under Section 43(2) of FSS Act, 2006 by FSSAI for appellate food sample testing. This has raised the total number of Referral Laboratories from 19 to 21 till date.
- As a step towards ease of doing business, FSSAI has developed a model document for Food categories and sub-categories along with the list of test parameters against each category and sub-category as per the FSSAI requirements to be used as a reference guiding material by the laboratories. This will bring uniformity in application and clarity for labs while applying for NABL accreditation under FSSAI-NABL integrated assessment.
- The Government of India has included Food Fortification including rice fortification under the National Nutrition Mission- POSHAN Abhiyan as a complementary intervention to reduce the prevalence of anemia and malnutrition in India. For effective implementation of the scheme, it becomes important to have a robust network of the Food Testing Labs that can be put in place for reliable and consistent testing of all the three fortificants, viz., Iron, Folic acid and Vitamin B12 as the quantum of fortified rice being supplied through the PDS would be in the range of about 20 Million Tons every year. In this direction, to have a uniform and standardized method for testing of fortificants in fortified rice across the country, FSSAI has standardized and approved the testing method for analysis of fortificants in Fortified rice. A grant of four crore (approx.) has been released to 26 States/UTs for procurement of equipment for testing rice fortificant and arranged training for the laboratory personnel. Eight batches of such training were scheduled for the participants across 29 SFTLs at EFRAC from July 2022.
- FSSAI has inaugurated the extension centre of Kolkata National Food Testing Laboratory (NFL) at Raxaul, Bihar to facilitate the imported food products from Nepal on 5th June, 2022. This lab also obtained NABL accreditation in 2 food categories. National Food Laboratory at Chennai Port Trust is ready for operationalization under PPP mode in collaboration with National Commodities Management Services Limited (NCML).
- FSSAI is implementing a Central Sector Scheme (CSS) for “Strengthening of Food Testing System in the Country Including Provision of Mobile Food Testing Labs” (SOFTeL) with financial outlay of Rs 613.69 Crore. A grant of Rs. 72.67 Crore has been released during the year to 31 States/UTs for procurement of High-end Equipment (including CAMC & Manpower for 7 years), Basic equipment, support for NABL, Rapid Kits, setting up of Microbiology testing facilities (with 24 equipment & 3 manpower for 3 years) etc. With this, a total Grant-in- aid of Rs.399.19 Crore has been sanctioned /released to 31 States/UTs for up gradation of 47 State Food Laboratories, including setting up microbiological laboratories. Process of setting up of Microbiology lab on turnkey basis has been started for 40 SFTL’s. FSSAI has initiated a project under the same scheme to install Laboratory Information Management System (LIMS) in FSSAI notified laboratories to bring the entire testing infrastructure under a single network.
- In 2022, FSSAI sanctioned grants for procurement of 175 Food Safety on Wheels (FSWs) to 23 States/UTs and grants for fuel and consumables to the extent of Rs. 10 lakh/per year per FSW vehicle for two years. These mobile food testing labs are being used by States/UTs for food testing, training and awareness generation, particularly in remote areas. The UT of Ladakh has procured one FSW and rest are under different stages of procurement through GeM portal. As of 31.10.2022, total 335 FSWs have been sanctioned to the 34 States/UTs out of which 161 FSWs have been procured or delivered to the States/UTs. A dedicated portal for monitoring of FSWs where the movement and performance of each FSW are being tracked has introduced.
- During the year, total 31 physical training programmes were organized by FSSAI in coordination with various Government and Non-Government Organizations/ Laboratories on safety parameters (mycotoxin, pesticides, heavy metals, microbiological parameters etc.) in different food commodities and Sensitization on Method Verification for Rice Fortificant Testing. 264 laboratory personnel (i.e. Food Analyst, Scientific Officer, Senior Technical Assistant, Junior Research Officers, Chemist, Microbiologist, Lab Technician etc.) from various State Food Testing Laboratories attended these training programs.
- Three NABL Awareness Programs on ISO/IEC 17025 Accreditation under Integrated Assessment for personnel from State Food Testing Laboratories and non-integrated Private Laboratories were held at Chennai (29.07.2022), Delhi (17.09.2022), Mumbai (21.10.2022) and Bengaluru (19.11.2022). Total 228 participants from various state and private labs participated in these programmes.
- During the year, total 141 training programs (Offline and Online) were organised by (i) ITC-FSAN, Mumbai (ii) Food Safety Solution Centre (FSSC), NFL Ghaziabad (iii) Centre of Microbiological Analysis Training (C-MAT), NFL Ghaziabad where 9640 participants from State Laboratories, Consumers, Students, Food Business Operators, Ministry of Micro, Small & Medium Enterprises (M/o MSME) & Food Start-ups, catering staff of INS, Food Safety Officers etc. availed the training programs on different areas related to food testing such as Determination of Melamine, Cyanuric Acid and Dicyandiamide in Dairy Products and Infant Formula by LC-MS/MS, Pesticide residue analysis in spices using GC-MSMS, Food Safety Risk Analysis, Be sure of Honey authenticity, Choosing the right Certified Reference Materials (CRM) for food safety, testing and analysis etc.
- FSSAI carried out the PAN-India Surveillance activities on Jaggery, Spices, Tea, Pulses, Milk & repeat surveillance on Trans- fats in selected foods in 2022. The Pan India Surveillance on Jaggery was carried out in selected 249 districts across 35 States and Union territories on 1st& 2nd February 2022 wherein 3060 samples of Jaggery variants were collected. Pan India Surveillance on Spices in 14 identified categories of whole or ground spices was conducted in selected 250 locations across 36 States and Union territories on 3rd & 4th March 2022 wherein 3582 samples were collected. The nation-wide tea surveillance was conducted in pre-selected 248 locations from 29 States and 7 Union territories during 27th& 28th April 2022 wherein 3251 samples of tea were collected. PAN-India Surveillance on Pulses was conducted on 4th – 5th August 2022, total 4676 samples of 10 variants of pulses (grams & dals) were collected. Conducted the Milk survey 2022 in 12 selected states in October 27th & 28th, 2022 due to the outbreak of Lumpy Skin Disease (LSD) in India. The survey was executed to assess the raw & pasteurized milk in 10 selected States (where there is prevalence of lumpy viral disease) and 2 states as control (where there is no outbreak of lumpy viral disease) for compliance of antibiotics/veterinary drugs, pesticide residues and heavy metals as given in FSSR. In all 795 samples were collected and the samples are under the process of analysis. The repeat surveillance on TFA was initiated on 9th – 16th November 22 in 550 districts. Packaged foods (10,484 samples) from identified 6 categories were collected from 550 selected districts. The collected samples are under the process of analysis. Authority has published Manual on (i) Methods of Analysis of Dairy and Dairy Products; (ii) General Guidelines on Sampling of Microbiological Analysis; and has published Methods on (i) Method for determination of niacin in foodstuffs; and (ii) Methods for determination of iron, folic acid and vitamin B12 in fortified rice.
- Ensuring quality of the fortified products, developed SOPs for FRK and FR manufacturer. Developed standards for FRK and premix of fortified rice kernel. Validation and proficiency testing of micronutrients in fortified rice, FRK and premix. FRK manufacturers are given license under 99.5 category which is a high-risk product and inspection is conducted prior to issuance of license to the FBO. Advisory was issued for all stakeholders related to food categories applicable for endorsement of + logo. Another advisory was issued related to category of license/registration of FRK manufacturers. Online and offline meetings were conducted with Ministries, State FDA, Education Department, Public and Civil Supplies division and WCD department to understand the current status and challenges in scaling-up of food fortification. Till date it has been conducted in 25 out of 36 States/UTs. Meetings were conducted with States/UTs to adopt food fortification in SNP and open market. Onsite training on Milk Fortification was provided at Srinagar. 4 private dairies have come forward to fortify their Milk. A virtual training on food fortification for State DOs/FSOs was conducted for their roles & responsibilities and challenges in the district. Till date it is conducted for 32 States/UTs and out of 2523 FSOs and 278 out of a total of 666 DOs have been certified. A training session was conducted on Eat Right India and food fortification for school teachers, medical consultants, MDM staff and food business operators, professional associations like IDA, IMA, IAPEN, AFSTi etc. A training session on food fortification was conducted for ASHA workers of West Bengal, Lakshadweep and Andaman & Nicobar Island.
- Awareness activity cum training was conducted in the districts of Jammu & Kahsmir, Ladakh, Puducherry, Himachal Pradesh and Sikkim in coordination with State FDA through CSR for MDM, ICDS supervisors, PDS beneficiaries, ASHA workers and college students. Food fortification stall and demonstration activity of fortified staples was integrated during Eat Right Mela organised by FSSAI in more than 35 cities across India. Further, awareness generation was done during malnutrition week in collaboration with Indian Association for Parenteral and Enteral Nutrition (IAPEN). 50+ activities like quiz competition, poster competitions, recipe competitions, webinar, health camps, health survey etc. were organised. Awareness through Print media was also done by publishing articles on food fortification regarding the need, benefits, staples fortified and myths.
- As part of the Eat Right India initiative, since January 2022, to promote safe, healthy and sustainable eating certifications have been completed under Eat Right India like Eat Right Campus (1230), Bhog (402), Clean Street Food Hub (79), Eat Right School (141), Clean and Fresh Vegetable Markets (93) and Eat Right Station (26). FSSAI organized “Eat Right Walkathon and Eat Right Melas” in cities across India to commemorate 75 Years of India’s Independence with support from the Food Safety Departments of various States. Since January 2022 107 Walkathons and Melas have been conducted have been conducted by FSSAI. Launched Eat Right Challenge for Cities and Districts to improve food environment in their respective areas in which a total of 188 Cities/ Districts participated and top 75 winners of this challenge were announced on 07th June 2022 on the occasion of World Food Safety Day. The second phase of Eat Right Challenge was also launched in April-2022 and the period of the challenge was from 1st May, 2022 to 15th-November, 2022 and a total of 221 districts have participated in it. FSSAI, in collaboration with the Smart Cities Mission, had launched the Eat Smart Cities Challenge to develop the entire urban food ecosystem for the participating cities to ensure safe, healthy and sustainable food for people. A total of 108 cities participated and top 11 cities were declared as winners and were facilitated on the occasion of World Food Safety Day, 7th June-2022.
- The Eat Right Creativity Challenge 3 was launched on the occasion of World Food Safety Day, 7th June-2022 for school children to tap the creative talent of students and enable them to inculcate healthy dietary habits. Challenge invites entries under three challenges on themes of 1. Eat Local and Seasonal for Poster competition 2. ‘Safer and healthy food for better future’ for Rangoli Competition 3. Benefits of Millets for Bulletin Board Competition on food safety from the Schools all over India and this challenge will culminate on 31st-decmeber-2022.
- Participated in various exhibitions and fairs to promote Food Safety and Hygiene and Eat Right India initiatives Ahaar-2022 in New Delhi, Global Patidar Business summit -2022 at Surat, Gujarat and Govt Achievements & Scheme Expo 2022, Pragati Maidan, New Delhi. Participated in India International Trade Fair -2022 from 14th Nov-2022 to 27th-Nov-2022 in Pragati Maidan New Delhi.
- FSSAI released books like Tadke Bina Zayka and Sugarless Desserts: Nature’s Sweetness in Every Bite. Tadke Bina Zayaka is a compilation of traditional Indian recipes without the use of visible fat. Sugarless Desserts: Nature’s Sweetness in Every Bite book is based on healthy dessert recipe that are prepared without the use of sugar or artificial sweeteners. These books have been released in the form of eBook for wider circulation.
- FSSAI extensively used its public awareness material through different social media platforms and promoted a variety of whole grains ranging besides wheat and rice to millets and other indigenous grains for better nutrition. A series of efforts are being undertaken to promote Millets as a nutri-cereal to sensitize citizens about our local and seasonal produce. In the year-2022 series of Recipe Raviaar various recipes made from different types of millets were promoted on social media. Monday millets has been launched as a part of promotion under United Nations’ declaration of “International Year of Millets-2023. Various Scroll Messages on Eat Right India and other necessary requirements related to licensing and registration process in Hindi, English and regional languages were telecasted on DD News, DD Kisaan and DD Regional Kendras from January 2022 to April 11, 2022. A campaign was also run on IRCTC on food safety, licensing and registration and Eat Right India to create consumer awareness. FSSAI reached the general public and various strata of society to sensitize on Eat Right India by announcing messages on All India Radio in Hindi, English and regional languages, through IRCTC website and its mobile app.
- Under FoSTaC initiatives, more than 3.09 lakh (till 25.11.2022) food handlers have been trained during 2022. Besides, through 1270 training programmes, FSSAI has provided Awareness training to more than 36,661 Street Food Vendors. Further, FSSAI had launched a 2-hour online training programme for food business operators exclusively on COVID-19 preventive guidelines. During the year, 336 Covid-19 trainings were conducted and more than 6556 persons were trained. More than 10 lakhs Food Handlers have been trained under this FoSTaC program during last 5 years. Under Induction/Refresher training programme till November, 2022, 195 Food Safety Officers, 132 Designated Officers and 257 Adjudicating Officers were trained.
- FSSAI is present at 61 entry points through offices located at 13 places i.e. Delhi, Mumbai, Kolkata, Chennai, Tuticorin, Hyderabad, Bangalore, Krishnapatnam, Vishakhapatnam, Kandla, Mundra, Kochi, Mangalore and Ahmedabad. At rest of the 97 points of entry, Customs Officers have been notified as Authorized Officers. To regulate and to ensure safety of food being imported in country especially the high risk food products like Milk, Egg, Meat, Infant Food, Nutriceuticals etc., it was decided that these products shall be only permitted through 61 points which are directly managed and manned by FSSAI offices. To facilitate trade, requirement of AGMARK certification for imported food consignments is suspended as Directorate of Marketing and Inspection (DMI) have no system of certifying Foreign countries under Agricultural Produce (Grading and Marking) Act, 1937. Various other steps have been taken towards shift in approach from end product testing to process certification system. A team of delegation from FSSAI visited Bhutan to carry out assessment of BAFR’s official food control system exercised on export of food products from Bhutan to India. MoU to be signed with BAFRA for recognition of their official control measures on food products exported from Bhutan to India. Registration of Foreign food manufacturing facilities intending to export milk, meat, egg power, infant food and nutraceuticals to India has been made mandatory as per FSS (Import) First Amendment Regulations, 2022.
- FSSAI continued to function as the National Codex Contact Point(NCCP) of India, and participate actively in the Codex work for development of International Standards that are fundamental to ensuring safety and fair practices in international trade of food products. During the year India participated in total 9 codex meetings viz. Fats and oils, fresh fruits and vegetables, spices and culinary herbs, fish and fishery products, contaminants, pesticides residues, veterinary drug residues, labelling, nutrition & food for special dietary use, food additives, Codex Executive Committee, Codex Alimentarius Commission. Further, India is actively involved as Chair/Co-chair and as a member in Development /Revision of standard for Ware Potatoes, Fresh Date, Chili Sauce, Mango Chutney, Guidelines on labelling of Non retail containers, Standard for Onion and Shallots, Revision of the Codex General Principles of Food Hygiene (GPFH) and its annex on HACCP and Internet Sales/E-Commerce; chilli pepper and paprika. During the year, in Codex Alimentarious Commission-45 meeting held in Rome, Italy, the proposals made by India in the interest of India on the following were adopted in different steps (i) Draft standard for fresh dates (at step 5/8); (ii) Proposal for new work on development of Standard for fresh curry leaves; (iii) Maximum level for cadmium in cocoa powder (100% cocoa solids on a dry matter basis) (at step 5/8); (iv) Draft Standard for dried floral parts – Saffron (at step 8); (v) Draft Standard for dried seeds – Nutmeg (at step 8); (vi) Draft Standard for dried or dehydrated chilli pepper and paprika (at step 5/8); (vii) Draft Standard for dried small cardamom (at step 5); (viii) Draft Standard for spices derived from dried fruits and berries (Part A – Allspice, Juniper berry, Star anise) (at step 5).
- At present FSAI has a sanctioned strength of 824, as on 31.10.2022, there are 311 regular employees in FSSAI out of which 77 officers are on deputation while 40 are on deemed deputation. In addition, 129 persons are on contract basis. Moreover, to fill PR posts, 80 posts of various levels have been advertised on 06.10.2022to fill on deputation basis. Process to fill 233 posts under Direct Recruitment against advt. no. DR-04/2021 is at various stages. CBT 1 and 2 of the advertisement has already been completed. Result for 45 posts of Assistants, IT Assistants, Hindi Translator and Junior Assistant Grade-I have also been declared. Results of remaining posts are expected to be declared by 31.12.2022. FSSAI has published Transfer Policy Guidelines on 01.11.2022 and also ratified the amendment in FSSAI (Salary, Allowances and other conditions of Service of Officers and Employees), Regulations, 2013. Draft FSSAI (Recruitment and Appointment) First amendment regulations, 2022 has also been published online for comments/suggestions.
- National Food Laboratory at JNPT, Mumbai has been established under PPP mode while one at Chennai Port Trust, Chennai under PPP mode is likely to be operational soon. To further streamline food imports, FSSAI opened new branch/port offices at Visakhapatnam, Krishnapatnam, Kandla, Mundra, Hyderabad, Bengaluru and Ahmedabad.
- Status and key achievements of National AIDS Control Programme for the Year 2021
- Introduction
- The Government of India is currently implementing the phase-V of the National AIDS and STD Control Programme (NACP). Like the previous phase, NACP phase-V is a Central Sector Scheme fully funded by the Government of India from 1st April 2021 to 31st March 2026 with an outlay of Rs 15471.94 crore. The NACP phase-V will anchor the national AIDS and STD response in the country till 2025-26 towards the attainment of the United Nations’ Sustainable Development Goals 3.3 of ending the HIV/AIDS epidemic as a public health threat through a comprehensive package of prevention, detection, and treatment services.
- NACP phase-V builds upon the game-changer initiatives undertaken during phase-IV including HIV/AIDS Prevention and Control Act (2017), Test and Treat Policy, Universal Viral Load Testing, Mission Sampark, Community-Based Screening, Transition to Dolutegravir-based Treatment Regimen etc. NACP phase-V introduces newer strategies consolidating and augmenting the gains to attain the stated goal by 2025-26.
- Prevention of new HIV infections
- The reduction of new HIV infections by 80% is the first goal of NACP phase-V. Continued and augmented focus on high-risk groups, bridge population and ‘at-risk’ populations is the mainstay of the NACP approach for the reduction of new HIV infections. Under the programme, more than one crore of high-risk groups, bridge and other vulnerable populations were covered in the first year of NACP Phase-V i.e., 2021-22. In 2022-23, till September 2023, around 55 lakh high-risk groups, bridge and other vulnerable populations were covered through 1476 targeted interventions and 156 Link Worker Schemes in the country.
- HIV prevalence among inmates in some States is higher than that among other HRG and bridge population groups. NACP phase-V calls for the universalization of the NACP interventions in prisons and other closed settings through a mix of service delivery models. In 2022-23, till September, NACP interventions offering both HIV and TB services, have reached 1538 institutions covering more than 5.50 lakh inmates. The programme has identified and provided treatment to more than 2200 HIV-positive cases and 400 TB cases in the group.
- Sampoorna Suraksha Kendras are being set up for providing services through a single window model for the HIV-negative ‘at-risk’ population across the prevention-test-treat-care continuum to keep them HIV-free. In 2022-23, the Sampoorna Suraksha Model has been piloted at 10 centres in three States of the country. Now, in the first phase, the programme is expanding the Sampoorna Suraksha Model to additional 65 centres across the 10 States of the country. The operational manual for the model has been drafted and the M&E framework for the same is being worked out.
- NACP phase-V does recognize the need to reach out to newer population groups to further expand the coverage of interventions, with a specific focus on the prevention of new HIV infections. Accordingly, it calls to develop and scale-up sustainable models for the ‘at-risk’ virtual population. NACO has recently released a White Paper on virtual interventions. The paper outlined the framework for virtual interventions with a specific focus on ethics, confidentiality and data security while indicating a potential mechanism for virtual outreach and service packages based on an extensive literature search.
- NACP has engaged with various partners to test various models of interventions on virtual platforms to reach out to the group. This includes Global Fund supported ‘NETREACH’, and PEPFAR supported ‘Safe Zindagi’, ‘Project Aspire’ and ‘Project Sunshine.
- Reducing AIDS-related mortality
- Reducing AIDS-related mortality by 80% is one of the five high-level goals of NACP phase-V. This would require 95% of HIV-infected people to know their HIV status, 90% to be on ART and 86% to be virally suppressed. NACP aims for accessible, affordable, and quality testing and treatment services as one of the building blocks for the attainment of reductions in AIDS-related mortality. Approaches of dual test kits (HIV & syphilis), dried-blood spot-based sample collection for viral load testing etc., will enhance the efficiency of the programme.
- In 2021-21, around 4.80 crore HIV tests were done under NACP including around 2.48 crore tests among pregnant women. In the first six months of 2022-23, around 3.10 crore HIV tests have been undertaken in the programme through various models. Given the trend, it is expected that more than 6 crore HIV tests would be undertaken during 2022-23 in NACP Phase-V. This would be the highest since the launch of HIV counselling and testing services under NACP in India.
- As on September 2022, around 16.24 lakh people living with HIV (PLHIV) were on anti-retroviral (ARV) treatment in the country. This is around 1.10 lakh more since September 2021 and the highest since the launch of ARV treatment under the National AIDS Control Programme in 2004. Most of them are on the most advanced, lifelong free Dolutegravir-based regimen which fast-tracks viral load suppression and significantly improves the quality of life.
- To monitor and improve the quality of care for HIV-infected people, the Government of India launched a free routine viral load test for PLHIV in February 2018. The number of viral load tests under NACP is continuously increasing. In 2018-19, around 2.50 lakh viral load tests were done. In 2021-22, around 10.32 lakh viral load tests were done which is more than 4 times the tests done in 2018-19.
- Quality laboratory services are critical enablers to the national AIDS and STI response with a central role across the prevention-detection-treatment cascade. The quality of laboratory services under NACP continues to be high. In 2021-22, among the laboratories and HIV counselling and testing centres participating in the quality assurance system, the discordance results were less than 0.10%. The six laboratories designated for early infant diagnosis scored a perfect 100% in the panel testing.
- The outcome of quality testing and treatment services being offered under the programme has been significant. As on March 2022, almost 18.56 lakh PLHIV were aware of their HIV status in comparison to the 16.99 lakh PLHIV in March 2019. The number of PLHIV on-ART increased from 13.99 lakh in March 2019 to 15.56 lakh in March 2022. The proportion of PLHIV who were on-ART and virally suppressed increased from 72% in 2018-19 to 85% in 2021-22.
- Elimination of vertical transmission of HIV and syphilis
- NACP phase-V calls for the attainment of the elimination of vertical transmission of HIV and Syphilis as one of the five top-level goals. This would require 95% of pregnant women are aware of their HIV and Syphilis Status. Till September 2022, almost 1.5 crore HIV testing among pregnant women was done against the target of 2.65 crore for the year. Around 1.2 crore Syphilis tests were done among pregnant women in the same period against the annual target of 1.91 crore.
- NACP is further developing a dedicated strategic roadmap to fast-track the progress on the elimination of vertical transmission. NACO has constituted a technical working group for a unified approach responding to both HIV and Syphilis. The strategic roadmap will review and update the strategies and operational flow in line with the NACP phase-V priorities.
- Universal access to quality management of sexually transmitted infections (STI)/ reproductive tract infections (RTI) services
- Sexually Transmitted Infections are indicative of ongoing unprotected high-risk sexual intercourse. For areas where HIV infection is not well established, the high prevalence of STI is an early warning of the epidemic potential of HIV from sexual transmission. Besides, one of the STIs, syphilis is of particular concern for maternal and child health given the commitment toward the elimination of congenital syphilis.
- Given the associations, the management of STI/RTI is one of the focus areas under NACP since its first phase. NACP phase-V has reinforced the focus on STI management by including it as one of the five high-level goals. Goal 4 of the NACP phase-V calls for the promotion of universal access to quality STI/RTI services to at-risk and vulnerable populations through a ten-prong strategy.
- NACP phase-V intends to augment the role of the Suraksha Clinics, the network of designated STI/RTI clinics delivering STI services following a syndromic management approach, to anchor newer initiatives like the Sampoorna Suraksha Strategy and integrated service delivery tailored to the local contexts. As a cross-cutting facility, these clinics, in their Sampoorna Suraksha avatar, will evolve into an integrated facility anchoring the country’s progress on the prevention of new infections, reduction of AIDS-related mortality, elimination of vertical transmission and promotion of access to STI/RTI services.
- In the second year of implementation, NACP phase-V aimed to manage 1.06 crore patients for STI/RTI. During April-September 2022, around 42 lakh clients were managed for STI/RTI episodes under the programme.
- NACO is leveraging improved laboratory technologies to enable equal access to integrated services for HIV and syphilis. NACP phase- V has adopted the rapid dual test kit for HIV & syphilis, with a specific framework on follow-up testing and treatment algorithms, increasing testing uptake in a very cost-efficient manner.
- Elimination of HIV/AIDS-related stigma and discrimination
- Building upon the gamechanger initiatives and in line with Government commitment to ‘Sabka Saath, Sabka Vikas, Sabka Vishwas, Sabka Prayaas’, NACP phase-V calls for the elimination of HIV/AIDS-related stigma and discrimination as one of the five top-level goals.
- The strategies adopted under the NACP have always kept the HRG and PLHIV at the centre of its response. With the notification of the HIV/AIDS (Prevention and Control) Act 2017 and the decriminalization of section 377 of the Indian Penal Code, the country has brought significant structural changes to eliminate HIV/AIDS-related stigma and discrimination.
- The Government of India has notified the HIV and AIDS Policy for Establishments 2022 which provides a framework to mitigate issues surrounding HIV and AIDS in a workplace setting and encourages action on part of the employer, employee and establishments to eliminate HIV-related stigma and discrimination. Additionally, nine Central Government Guidelines under the HIV and AIDS (Prevention & Control) Act, 2017 have also been notified. Twenty-seven States/UT has appointed an ombudsman at the State level who is responsible to handle cases of HIV-related stigma and discrimination.
- Break the silos, build synergies
- ‘Break the silos, build synergies’ is a guiding principle of the NACP phase-V. This asks for the offering of a basket of tailored integrated services across the prevention-detection-treatment spectrum. This means establishing functional referrals and linkages for the management of relevant communicable and non-communicable diseases, sexual & reproductive health, and mental health through coordinated systems.
- NACP has made significant progress in engagement with National Health Mission, National Viral Hepatitis Control Programme and National Viral Hepatitis Surveillance Programme while strengthening the collaboration with National Tuberculosis Elimination Programme. Hepatitis B and Hepatitis C have been integrated as additional biomarkers in our robust Surveillance Systems. NACO is also working with the Ministry of Social Justice and Empowerment for an integrated package of service delivery through linkages for people who use drugs.
- NACP phase–V has augmented the systematized convergence with the existing schemes of the Central Government including synergy with National Health Programmes and related ministries. So far, the Memoranda of Understanding have been signed with 18 key departments and ministries of the Government of India. Taking the national AIDS response beyond the prevention-testing-treatment spectrum, the programme has engaged with State Governments and other stakeholders to offer social protection schemes for the infected and affected. The State AIDS Control Societies have engaged with key departments so that the affected population are linked with various social protection schemes addressing inequalities and facilitating a dignified life. Twenty-six States and UTs are providing financial support to the people infected with HIV and to their families.
- Evidence-driven response
- NACP phase-V has continued to augment and harness its robust strategic information systems of programme monitoring, surveillance & epidemiology, and research & evaluation for evidence-led interventions improving the directions and results under the programme.
- The integrated and enhanced surveillance and epidemiology have provided crucial evidence on the level and trends of HIV, Syphilis and related co-infections of Hepatitis B and Hepatitis C in eight population groups. The evidence thus generated has helped the programme to build clusters in each State of the country to anchor the AIDS response through the District Integrated Strategy for HIV/AIDS (DISHA). In July 2022, NACO launched the DISHA strategy revamping the district-level monitoring and implementation mechanism under NACP Phase-V.
- To strengthen evidence-led planning and implementation, NACO has institutionalized operational research at the State level. Further, NACP brought in newer evidence through two national consultations and undertook pilots on newer concepts such as pre-exposure prophylaxis, HIV interventions for at-risk populations in virtual spaces, and HIV self-testing.
- Dissemination is one of the core functions of strategic information management under NACP. The national technical guidelines on pre-exposure prophylaxis and the White Paper on strategies for engaging with HIV at-risk populations in virtual spaces have been released. On the occasion of World AIDS Day 2022, NACP has further released the fourth edition of the ‘Sankalak-Status of the National AIDS response’, and the technical reports from the 2021 round of HIV surveillance and estimations.
- Impact
- As a result of strong political leadership, evidence-led policies and strategic implementations, the national AIDS response has been highly successful. As per the latest HIV Estimates of the Government of India, HIV prevalence in India continues to be low with an adult prevalence of 0.21% in 2021. The annual new HIV infections have declined by 46% between 2010 and 2021, against the global average of 32%; and the AIDS-related mortalities have declined by 76%, against the global average of 52%.
- E-Health
National Telemedicine Services
The National Telemedicine Service “eSanjeevani” is a digital health initiative of the Ministry supports two types of teleconsultation services-Doctor-to-Doctor (eSanjeevani) and Patient-to-Doctor (eSanjeevani OPD) Tele-consultations. eSanjeevani was rolled out in November 2019 as an important component of the Ayushman Bharat Health and Wellness Centre (AB-HWCs) programme. It aims to implement tele-consultation in all the 1.5 lakh Health and Wellness Centres in a ‘Hub and Spoke’ model, by December 2022. NHM in States identify and set up dedicated ‘Hubs’ in Medical Colleges and District hospitals to enable tele-consultations services to ‘Spokes’, set up at SHCs and PHCs.
In wake of COVID 19 Pandemic, on the 13th April 2020, the MoHFW rolled out ‘eSanjeevaniOPD’ first of its kind to facilitate online health services to patients directly in the confines of their home at no cost to ensure continuity of care.
eSanjeevani has completed around 7.7 crore consultations. Over 3,50,000 patients are seeking health services on a daily basis in 36 States/UTs. The top ten States which have registered highest consultations through eSanjeevani and eSanjeevaniOPD platforms are Andhra Pradesh (27853960), West Bengal (9813829), Karnataka (9166769), Tamil Nadu (8349601), Maharashtra (3966846), Uttar Pradesh (3637856), Madhya Pradesh (3081029), Bihar (2575590), Telangana (2136862), Gujarat (1608160).
Ayushman Bharat Digital Mission (ABDM): In year 2019, Ministry of Health and Family Welfare released National Digital Health Blueprint (NDHB) as an architectural framework for effective implementation of Digital Health interventions.
With a vision to create a national digital health ecosystem as proposed in NDHB, on 15th August 2020, Hon’ble Prime Minister Shri Narendra Modi announced the launch of the National Digital Health Mission (now known as Ayushman Bharat Digital Mission) in six union territories (Andaman & Nicobar Islands, Chandigarh, Dadra & Nagar Haveli and Daman & Diu, Lakshadweep, Ladakh and Puducherry) on pilot basis. Three key registries of NDHM namely Health ID, Health Professional Registry (HPR), Health Facility Registry (HFR) and digital infrastructure for data exchange have been developed and implemented in these UTs.
On 27th September, 2021, the Hon’ble Prime Minister announced the nationwide rollout of the Ayushman Bharat Digital Mission (ABDM) (earlier known as National Digital Health Mission) with aims to develop the backbone necessary to support the integrated digital health infrastructure of the country.
- National Centre for Disease Control (NCDC)
National Centre for Disease Control (NCDC) has its headquarters in Delhi and has 8 branches located at Alwar (Rajasthan), Bengaluru (Karnataka), Kozhikode (Kerala), Coonoor (Tamil Nadu), Jagdalpur (Chhattisgarh), Patna (Bihar), Rajahmundry (Andhra Pradesh) and Varanasi (Uttar Pradesh).
The technical Centres/Divisions at the headquarters of the institute are:
- Integrated Disease Surveillance Programme (IDSP)
- Division of Epidemiology
- Division of Microbiology
- Division of Biotechnology and Viral Hepatitis
- National Program for Surveillance of Viral Hepatitis
- Division of Parasitic Diseases
- Centre for Arboviral & Zoonotic Diseases
- Division of Zoonotic Disease Programme,
- Centre for Environmental & Occupational Health, Climate Change & Health
- Centre for Non-Communicable Diseases
- Integrated Disease Surveillance Programme (IDSP)
IDSP covers all states and UTs with the objective to strengthen/maintain decentralized laboratory-based IT enabled disease surveillance system for epidemic prone diseases and to monitor disease trends to detect and respond to outbreaks in early rising phase through trained Rapid Response Team (RRTs). IDSP has also been coordinating the overall surveillance activities in India regarding CoVID – 19 pandemic. CSU, IDSP has been assisting the States/UTs in their epidemiological investigation and containment.
- International Health Regulations
NCDC is designated National Focal Point for India. India has declared itself IHR compliant in July 2016. Functions of NFP include: capacity building for IHR(2005) in the country, review progress of IHR implementation by using WHO IHR monitoring tool and share with WHO annually, coordination and communication with WHO, NFP of other countries and local stakeholders for event verification, notification, contact tracing(TB), etc.
- INSACOG:
- The Indian SARS-CoV-2 Genomics Consortium (INSACOG) is a national multi-agency consortium of Genome Sequencing Laboratories (RGSLs) laboratories established by the Government of India on 30th December 2020.
- The network carries out whole genome sequencing of SARS-CoV-2 virus across the nation, aiding the understanding of how the virus spreads and evolves, and provides information to aid public health response.
- A summary of the cumulative data of INSACOG and other state sequencing initiatives can be found in the INSACOG data portal along with other INSACOG related information at https://ibdc.rcb.res.in/
- Presently, INSACOG is keeping a close watch and monitoring the emergence and evolution of XBB and XBB.1 and any new sub-lineages.
Status of Variants and Sequencing as on 09th September 2022:
- Total samples sequenced: 2,86,860
- INSACOG samples with PANGO lineage assigned: 1,86,468
- Total VOCs: 1,52,327
- Division of Biotechnology and Viral Hepatitis
The division provides molecular diagnostic services, molecular epidemiology, specialized training and applied research on various important epidemic-prone diseases of public health importance. The following activities were performed :
- Whole Genome Sequencing of 9541 positive samples of SARS CoV-2, using Next Gene Sequencer, Illumina machine as a hub lab under INSACOG. All of the sequences are continuously being uploaded on the GISAID database.
- SARS CoV -2 RT PCR test to diagnose COVID 19 in 2,547 (Till January, 2022) samples using the Roche Cobas 6800 automated system.
- Targetted sequencing of 190 samples for HBV, CCHF, Lyssa virus, Kala Azar, Toxoplasma, Zika, Rabies and Yellow Fever using Sanger Sequencer.
- Viral load estimation of 403 Hepatitis B and 727 Hepatitis C seropositive samples.
- Confirmation of 54 HCV samples using conventional PCR.
- Capacity building by imparting four days training on Whole Genome Sequencing using Illumina 550 platform was provided to 4 participants (Medical Professors) from Gandhi Medical College, Bhopal, M.P.
- Lab strengthening by installation and training for newly purchased Applied Biosystems 3500 XL Genetic Analyzer.
- Training of students on various molecular techniques in the division with five students completing their Masters dissertation.
- National Program for Surveillance of Viral Hepatitis
National Program for Surveillance of Viral Hepatitis is a central sector scheme under Umbrella Scheme of National Centre for Disease Control with the objective of strengthening the existing infrastructure for establishing surveillance of viral hepatitis and estimating burden of viral hepatitis infection in India
Achievements
Laboratories of 15 medical colleges/institutions strengthened across the country to carry out surveillance of viral hepatitis. Program has expanded its network and included a total of 30 laboratories in FY 2021-22 as compared to 15 in FY 2020-21. Viral Hepatitis Laboratory at NCDC has been designated as Centre of Excellence under the program and is involved in testing of referral samples for biomarkers for hepatitis for both serology and viral load. The program division is in the process of obtaining NABL accreditation of the Viral Hepatitis laboratory at NCDC.
- Surveillance of Acute Viral Hepatitis
- Activity for surveillance of acute viral hepatitis initiated in 2021 based on Operational guidelines and strategies developed by technical resource group comprising of public health experts, microbiologists, clinicians, program managers from across the country. Activities for Surveillance of acute viral hepatitis started since January 2021 in earlier 15 regional sites and May 2022 in new 11 regional sites
- Training Module developed and capacity of human resource involved in the activity built
- Surveillance of Chronic Viral Hepatitis-Integration with population level surveys/ programs
- Integration with National Family Health Survey (NFHS)-4 and National Family Health Survey (NFHS)-5
- Integrated with ICMR, IIPS Mumbai, ICMR NARI Pune and statistics division, MOHFW for inclusion of markers for hepatitis B and C in NFHS-5 & NFHS-6 for estimating
- Seroprevalence of hepatitis B and hepatitis C in adult population in India based on NFHS-5 and NFHS-6
- Estimating the SDG indicator 3.3.4 by testing of biomarker for hepatitis B in children <5 years of age.
- Currently, testing of specimens collected during NFHS-5 is in process.
- Integration with HIV Sentinel Surveillance (HSS) of National AIDS Control Program (NACP)
- Inclusion of bio-markers of hepatitis B and C in HSS utilising existing machinery of HSS for optimal utilisation of resources. Analysis of data of 17th round of HSS plus is ongoing. The program has also integrated for testing of biomarkers of hepatitis B and hepatitis C in the next round of HSS planned in last quarter of FY 2022-23 so as to obtain the seroprevalence of hepatitis B and hepatitis C in general population as well as in high-risk groups
.
- Integration with National Viral Hepatitis Control Program (NVHCP)
- Establishing linkages and mechanisms for follow-up of individuals detected positive on screening for hepatitis B/C for counselling, confirmatory testing and linkages to care and support services to high risk groups, prison inmates and pregnant women and their newborns.
- Department of Parasitic Diseases (DPD)
Deals with the activities related to Neglected Tropical Diseases namely Soil Transmitted Helminthiasis (STH), Guinea worm Disease and Lymphatic Filariasis. NCDC functions as the National Nodal Agency for implementation and monitoring of the National Guinea Worm Eradication Programme. The Department also functions as National Nodal Agency for prevention and control of STH in the country and has been continuously monitoring the STH burden in the country through periodic prevalence assessment surveys.
- Centre for Arboviral & Zoonotic Diseases
Deals with the Zoonotic diseases of public health importance including outbreak prone and emerging infectious diseases mainly Plague, Rabies, Kala-azar, Arboviral infections (Dengue, JE, Chikungunya, Zika virus & CCHF) Toxoplasmosis, Brucellosis, Leptospirosis, rickettsiosis, hydatidosis, neurocysticercosis, SARS Cov2, and Anthrax. The role of division is primarily to provide laboratory evidence by conducting special and reference level tests which are not available at most of the institutes or medical colleges in India.
- Division of Zoonotic Disease Programme
Nodal agency for implementation of three central sector schemes of National health program for Rabies, Leptospirosis and Intersectoral coordination program for prevention & control of zoonotic diseases.
- Centre for Environmental, Occupational Health and Climate Change & Health
This centre addresses the health-related issues pertaining to climate and environmental factors. After introduction of “Mission on Health” in year 2015 under the Prime Minister’s Council on Climate Change (PMCC), India’s National Action Plan for Climate Change and Human Health (NAPCCHH) was prepared and to implement it National Programme on Climate Change and Human Health (NPCCHH) under National Health Mission (NHM) was approved by MoHFW in February 2019; this centre looks into NPCCHH operations in the country. Key objectives are increasing awareness, building capacity of health professionals, strengthening health systems and infrastructure for response – health adaptation plans, vulnerability assessments, surveillance, EWARS, etc, building collaborations and research & innovations in context of climate change and human health.
- Centre for Non-Communicable Diseases
In response to the increasing burden of non-communicable diseases, Centre for Non-Communicable Diseases (NCDs) was set up in February 2015, in NCDC with the objectives of providing technical support to NPCDCS, capacity building, IEC & advocacy with policy makers & NPCDCS programme managers, monitoring & evaluation and research.
National Programme for Prevention & Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS)
In order to prevent and control major NCDs, the Government is implementing the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS) with focus on strengthening infrastructure, human resource development, health promotion, early diagnosis, management and referral.
Under NPCDCS, 707 District NCD Clinics, 193 district Cardiac Care Units, 268 District Day Care Centre, 5541 CHC NCD Clinics have been setup across the country.
A population based initiative for prevention and control, screening and management initiative
for common NCDs (Diabetes, Hypertension and common cancers viz. Oral, Breast and Cervical Cancer) has been rolled out in the country under NHM and also as a part of Comprehensive Primary Health Care. Under the initiative, persons more than 30 years of age are targeted for their screening. This is done at all Ayushman Bharat – Health and Wellness Centres (HWCs). Till 22.12.2022, more than 1,37,118 HWCs have been operationalized out of the target of 1.5 lakhs throughout India. The number of persons screened so far through Health and Wellness Centres includes 29.89 Cr for hypertension, 25.50 Cr for diabetes, 17.40 Cr for oral cancer, 8.25 Cr for breast cancer and 5.65 Cr for cervical cancers. Training Modules have been developed for training of various categories of health staff viz. MOs,Nurses, ANMs and ASHAs. About 29,632 Medical Officers, 80,418 Community Health Officers, 29,076 Staff Nurses, 2.25 lakhs ANM/Multi-Purpose Workers and 7.19 lakhs ASHAs have been trained.
- Special Days pertaining to NCD like National Cancer Awareness Day (7th November), World Diabetes Day (14th November), World Cancer Day (4th February), World Kidney Day (10th March) are observed through different social media handles of the Ministry.
Under the Strengthening of Tertiary Cancer Centre facilities Scheme of NPCDCS, financial assistance is being provided for setting up of State Cancer Institutes (SCIs) and Tertiary Care Cancer Centres (TCCCs) in different parts of the country. Under the programme, 19 SCIs and 20 TCCCs have been approved to provide advance cancer care services. The support to the States for the centrally sponsored components will be provided only for period up to 3lst March 2024, implying that all the approved projects under the various tertiary care programme would need to be completed by 31st March 2024.Seven institutes are completed and other 32 institutes are at different stages of development.
National Cancer Registry Programme (NCRP): It is in existence since 1982. Now under National Centre for Disease Informatics and Research (ICMR) since 2011.NCRP functions through Population and Hospital Based Cancer Registries (PBCR and HBCR) across different states in India. 38 PBCRs and more than 189 Hospital Based Cancer Registries have been registered under NCDIR –NCRP. These different registries are at different geographical locations. Deals with various cancer sites in the body.
Pradhan Mantri National Dialysis Programme (PMNDP) has been implemented in total 36 States/UT in 630 Districts at 1339 centres by deploying 8608 hemo-dialysis machines. Total 16.17 lakh patient availed dialysis services and 174.78 Lakhs Hemo-dialysis sessions held- as on 31st October 2022. Hon’ble Union Minister of Health & Family Welfare Dr Mansukh Mandaviya launched PMNDP portal on 5th May 2022. It is integrated with ABHA ID. The portal will integrate all the dialysis centres operational in the States/UTs under NHM with building of registry and facilitating portability throughout the country (One Nation One Dialysis).
National Multi-sectoral Action Plan (NMAP) has been developed in consultation with 39 departments of Union Government and other stakeholders for prevention and control of NCDs. The plan offers a road map and menu of policy options to guide multi-sectoral efforts towards attaining the NCD targets mentioned in the National Health Policy, 2017 and National NCD Monitoring Framework. An Inter-Ministerial Committee (IMC), to coordinate the multi-sectoral actions has been setup. Nodal Officer has been appointed in line ministries for NMAP. Series of meetings including of IMC have been conducted. Last meeting was conducted on 19th October 2021 under the chairmanship of Secretary (Health & FW).
India Hypertension Control Initiative (IHCI): To leverage and strengthen the ongoing efforts of hypertension control interventions by NPCDCS under NHM and improve the linkages between population-based screening initiative with health care, India Hypertension Control Initiative (IHCI), a collaborative project of ICMR, MoHFW, State Governments and WHO has been implemented in 141 districts in 25 states. Till 31st October 2022, IHCI has been implemented in nearly 21579 health facilities with over 3596982 hypertension patients and 1479621 diabetes patients.
- National Viral Hepatitis Control Program (NVHCP)
National Viral Hepatitis Control Program under the National Health Mission in alignment with SDG 3.3 aims to target the management of 5 crore people possibly harbouring the infection. Under the program, free diagnostics and drugs are being made available to all in need, not only for treatment of hepatitis C, but also for life-long management of hepatitis B. The key strategies adopted under the program include preventive, promotive and curative interventions with the focus on awareness generation, increasing access, promoting diagnosis and providing treatment for viral hepatitis. During 2018–22(till Sept 2022), it benefitted nearly 4.44 crore individuals and treated more than 1,74,000 patients of viral hepatitis
Currently, services for the diagnosis and treatment of viral hepatitis are available in all the states and UTs. The programme has collaborated with the National Program for Surveillance of Viral Hepatitis (NPVSH), National AIDS Control Program and Immunization Division. NVHCP has a national helpline (1800-11-6666) along with the TB helpline to provide access to information about viral hepatitis and services under the programme. The Programme is also in process of collaborating with other existing programs such as RMNCHA+N etc. for effective implementation of the program. The programme has the paperless data recording & reporting on NVHCP Management Information System for robust Monitoring & Evaluation.
Achievements (April – September, 2022):
- 868 treatment sites established for management of viral hepatitis across 705 districts.
- Serological tests done for diagnosis of viral hepatitis C: 38,39,014
- No. of patients initiated on treatment of Hepatitis C : 35,596
- Patients who have completed the treatment of HCV (end of treatment): 19,643
- Serological tests done for diagnosis of viral hepatitis B: 1,04,43,109
- No. of patients initiated on treatment of Hepatitis B: 10,324
Viral Hepatitis Laboratory
- Viral Hepatitis Laboratory is involved in testing of all serological and molecular markers of viral hepatitis. It is involved in detection & investigation of outbreaks and describing trends in type-specific acute hepatitis. The laboratory is involved in carrying out outreach screening of biomarkers of hepatitis B and hepatitis C. In 2022, screening camps have been organized in district Amroha, Bijnor, Baraut and Ghaziabad. Total samples tested in 2022 are 7412 (approx.) of which 6658 samples were tested as a part of outreach screening camps.
- The laboratory has been designated as the apex laboratory for National Viral Hepatitis Control Program and as Centre of Excellence for National Program for Surveillance of Viral Hepatitis.
- The laboratory is in the process of obtaining NABL accreditation. The laboratory has participated in External Quality Assessment scheme for acute hepatitis serology, hepatitis B Serology and Blood borne viruses (HBsAg and HCV). The laboratory has also participated in Inter-laboratory comparison (ILC) with Institute of Liver and Biliary Sciences for viral loads for hepatitis with 100% concordance.
- Central Government Health Services (CGHS)
CGHS is a health scheme mainly for serving/ retired Central Government employees and their families. The scheme was started in 1954 in Delhi. The scheme expanded in the scope of services as well as coverage across the country over a period of time.
- The scheme was extended from 25 in 2014 to 77 cities – through 336 Allopathic Wellness Centres and 97 AYUSH Centres /Units. It serves 14.72 Lakh Primary Cardholders and 41.92 Lakh beneficiaries (2022) expanding from 10 Lakh primary Card holders and 34 Lakh beneficiaries in 2014.
- Number of CGHS WELLNESS CENTRES / UNITS
- No of Allopathy Wellness Centres has increased from 254 in March, 2014 to 336 in Oct, 2022.
- No of AYUSH Wellness Centres/Units has increased from 85 in March, 2014 to 97 in March, 2022.
- Drug Regulation
- G.S.R. 20(E) dated 18.01.2022 for incorporation of QR coding on packing of Active Pharmaceutical Ingredients (APIs) for tracking and tracing in the supply chain under Drugs Rules, 1945.
- G.S.R. 356(E) dated 18.05.2022 to insert new rule 43A under Medical Devices Rules, 2017 for Suspension and cancellation of license of import licence.
- G.S.R. 450(E) dated 15.06.2022 to amend the requirement of Transmissible Spongiform Encephalopathies (TSEs) or Bovine Spongiform Encephalopathy (BSE) certificate in the Fourth Schedule under Medical Devices Rules, 2017.
- G.S.R. 654(E) dated 24.08.2022 for parallel submission of application for Marketing Authorisation and grant of manufacturing licence in Form 28-D under Drugs Rules, 1945.G.S.R. 754 (E) dated 30.09.2022 to regulate sale and distribution of regulated medical devices by system of registration of premises and seller or distributor, involved in it under Medical Devices Rules, 2017.
- G.S.R. 777(E) dated 14.10.2022 regarding exemption of certain Class A medical device from licensing regime under Medical Devices Rules, 2017.
- G.S.R. 778(E) dated 14.10.2022 for streamlining of regulatory process for strengthening the ecosystem to boost innovation by providing for deemed approvals for various permissions under New Drugs & Clinical Trials Rules, 2019.
- G.S.R. 823(E) dated 17.11.2022 regarding amendment in rule 96 of Drugs Rules, 1945 for mandating Bar code or QR code on the label of top 300 pharmaceutical brands available in Indian market as test trial on voluntary basis by the manufacturers.
- DENTAL COLLEGES
At present, there are 318 dental colleges in the country, out of which 51 are in Government Sector and 267 in private sector with annual admission capacity of about 27,868 Under Graduate seats and 6,823 Post Graduate seats. Under EWS scheme promulgated under 103rd constitutional amendment, 2019, the DCI has recommended 468 additional seats at UG level at 31 Govt. Dental Colleges and 47 PG Seats at 16 Govt. Dental Colleges for the academic session 2022-23.
Reforms in Dental Education:
The Draft National Dental Commission Bill
The Government has initiated a series of reforms in the sector of medical education over the last six years. As part of these reforms and in order to revamp the Dental Education System in the country to bring it at par with global standards, a draft National Dental Commission Bill (the Bill), to replace the existing Dentists Act, 1948, has been prepared and was placed in public domain on 28.01.2020 for inviting comments of State Governments, general public and other stakeholders. The 2372 suggestions/comments received from various stakeholders have been examined by a Committee of Dental Experts under the Chairpersonship of Chief of the Centre for Dental Education & Research, AIIMS, New Delhi. The suggestions of the committee have been duly considered and incorporated appropriately in the Bill.
The Bill provides for the constitution of a National Dental Commission (the Commission), four Autonomous Boards, a Dental Advisory Council and State Dental Councils. The Commission shall be a corporate body. The Bill further provides for constitution of four Autonomous Boards namely Undergraduate Dental Education Board (UGDEB), Postgraduate Dental Education Board (PGDEB), Dental Assessment and Rating Board (DARB) and Ethics and Dental Registration Board (EDRB) – to regulate the education, examination, training and services of dental professionals and dental auxiliaries like dental hygienists, dental technicians and dental operating room assistants. A provision for National Dental Advisory Council has also been kept in the Bill to have representation of States/UTs at National level. The Bill also provides for creation and maintenance of digital and live National and State Registers of all licensed dental professionals and dental auxiliaries.
As per the Bill, the Commission shall lay down policies for maintaining high standards in dental education, and shall also exercise appellate jurisdiction with respect to the decisions of the Autonomous Boards. The Commission shall frame guidelines and conduct a uniform entrance test at undergraduate level i.e. National Eligibility cum Entrance Test, a uniform exit test i.e. National Exit Test (Dental), common counselling for admission to undergraduate and postgraduate dental courses etc.
The Bill empowers the Central Government to give directions to the Commission and the Autonomous Boards on questions of policy and also to the State Governments for carrying out all or any of the provisions of this Act.
The draft Bill is still under consideration.
- National Programme for Health Care of the Elderly (NPHCE)
- The Government of India had launched the “National Programme for Health Care of the Elderly” (NPHCE) during 2010-11, to address health related problems of elderly people and to provide separate, specialized and comprehensive health care to the senior citizens at various levels of state health care delivery system including outreach services. Preventive and promotive care, management of illness, health manpower development for geriatric services, medical rehabilitation & therapeutic intervention and IEC are some of the strategies envisaged in the NPHCE. During 2022-23, a total number of 725 districts of 35 States/UTs and 19 Regional Geriatric Centres (RGCs) and 02 National Centres for Aging (NCAs) are sanctioned under the programme. Of the 725 districts sanctioned,640 district hospitals & 18 RGCs have operationalised for NPHCE services. The programme implementation at States/UTs level is through funds under NCD Flexible pool of National Health Mission (NHM) and at Government Medical Colleges/Institutes through funds under Tertiary care programme activities of Ministry of Health & Family Welfare.
- Progress in Operationalization of the Programme activities 2022-23 upto nov. 2023:-
| S. No | Institutions | Sanctioned | Operational
|
||||
| OPD | Indoor wards | Physiotherapy services | Laboratory services
|
||||
| 1 | RGCs | 19 | 18 | 16 | 14 | 14 | |
| 2 | District hospitals | 725 | 640 | 525 | 525 | 540 | |
| 3 | CHCs | 4888 | 3458 | – | 1372 | – | |
| 4 | PHCs | 18651 | 11674 | – | – | – | |
| 5 | SCs | 90932 | 14320 | – | – | – | |
- Daily Geriatric OPD services are being provided in 640 DH,3458 CHCs and 11674 PHCs along with special OPDs in 18 RGCs. Inpatient services are being provided in 525 DH, along with 17 RGCs. Physiotherapy services are being provided in 525 DH, 1137 CHCs along with 14 RGCs.Laboratory services are being provided in 540 DHs, 1372 CHCs, along with 14 RGCs.
- 1stOctober-International Day for Older Persons:-Every year 1st October is celebrated as the International Day of Older Persons to recognize the important contributions that older people make to our world, while raising awareness towards issues of aging. The theme of International Day of Older Persons (UNIDOP) 2022 – The Resilience and Contributions of Older Women – serves as an opportunity to delve into the opportunities and challenges and frame solutions with resilience and fortitude. Considering that the needs of elder women are less prioritized and multi-stakeholder engagement is imperative to achieve Healthy Ageing, Ministry of Health and Family Welfare, Government of India, in collaboration with WHO India, organized a two-day national consultative workshop – Multi-stakeholder Engagement for Active & Healthy Ageing among Elderly Women on 21-22 October, in New Delhi, to address initiatives in various sectors for elderly with a focus on elderly women. The consultative workshop witnessed participation of international, national and state level speakers. The event brought together practitioners, administrators, policy makers, implementers, researchers, academicians and civil society organizations in the field of health of older people to share their experiences and collaborate towards an equitable agenda on older people, especially elderly women.
- Activities Conducted by States/UT to commemorate International Day for Older Persons:-
- Month long campaign to conduct comprehensive geriatric assessment and care at old age homes and DH/CHC/PHC level.
- Health Camps for elderly with COVID protocols and Distribution of Aids procured through the social welfare department.
- Awareness Programme on Healthy Ageing, NCD Screening, Demonstration of Physical exercise/Yoga, Distribution of Elderly Diaries, Counselling sessions etc.
- Felicitation of the elderly person in various States including NGOs & Institutions.
- Awareness generation through Hoardings at prominent places, wall paintings, posters, pamphlets, Radio programmes, announcements, News Paper Advertisement, community programs through IEC van.
- Sensitization /workshop for all working staff for implementation of NPHCE programme at District and CHC level.
- Training Modules: Three sets of Training modules for Medical Officers, Nurses and Community based workers to deliver Comprehensive Geriatric Assessment & Care have been developed and shared with all States/UTs. The State level Training of Trainers of Medical Officers & Nurses for Comprehensive Geriatric Assessment & Care is being conducted.
- IEC:-Audio/Video spots on different topics of elder care, print material-folder, posters etc. have been developed. The regional language version of IEC material is being developed. https://nphce.nhp.gov.in/video-spot/
- In (IITF) 2022 India International Trade Fair Elderly people above 60 years were provided mental Health counselling services by Psychiatric, counsellors and Psychiatric Social worker of RML Hospital and Lady Harding Medical College.
- National Oral Health Programme (NOHP)
National Oral Health Programme (NOHP) has been launched in 2014 to strengthen the public health facilities of the country for an accessible, affordable & quality oral health care delivery. Under the NHM Component of the Programme, support is provided to States to establish dental care unit at the level of the district hospital or below. The Programme is functional across 36 States/UTs and till now 4750 dental care units have been supported under the Programme in the States/UTs. The Central Component of the Program looks after the overall planning, implementation, capacity building and monitoring and evaluation of the implementation of the Program in all States/UTs. CDER, AIIMS was designated as Centre of Excellence for implementation of NOHP. The following National Resource Centres have been established under NOHP to facilitate research, training and capacity building in the identified areas: –
- Tobacco Cessation and Oral Health at MAIDS, New Delhi
- Oral health of children and elderly at PGIMER, Chandigarh
Oral health manuals for school teachers and healthcare workers has been developed in English and Hindi. Oral Health trainings were conducted for AYUSH professionals, school teachers and nurses. TV commercial, radio jingle, posters and pamphlets for oral health have been developed and being used for mass media awareness campaign. Toll free National Dental and Oral Health IVRS Helpline developed and launched in English and Hindi (Toll Free Helpline – 1800-11-2032). e-DantSeva, an interactive website and mobile application for oral health has been initiated. Mapping of all dental facilities in the country is on way to facilitate service seeking by the public. Reference manual for Tobacco Cessation for dentist has been developed to facilitate tobacco cessation in dental settings. For oral health care of the elderly, “Reference Manual for Caregivers on Oral Healthcare of Elderly” has been released